the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Wood vinegar for sheep receiving high-concentrate diets
Vitor L. de L. Melo
Thiago L. A. C. de Araújo
Patrícia de O. Lima
Salenilda S. Firmino
Pedro P. N. de Oliveira
Alexandre S. Pimenta
Michelly F. Macedo
José G. L. de Almeida
Antônia V. de A. F. Amâncio
Maiko R. T. Dantas
Dorgival M. de Lima Júnior
The antibacterial, antifungal, and anti-inflammatory properties of refined wood vinegar make it a promising product for ruminant nutrition. This study aimed to evaluate the effect of increasing oral doses (0, 10, 20, 30, and 40 mL d−1) of wood vinegar (WV) on the intake, apparent digestibility, ingestive behavior, water balance, ruminal parameters, serum biochemistry, nitrogen balance, and physiology of sheep fed with high levels of concentrate. We used five castrated male sheep, Dorper × Santa Inês, with an average age of 36 months and an average weight of 59.34±5.73 kg, in a Latin-square design. The animals were provided a total mixed ration formulated at a roughage-to-concentrate ratio of 20:80, which was offered twice daily following the delivery of half the daily dose of WV. There was no effect (P>0.05) of WV on dry-matter intake. Increasing WV levels linearly increased (P<0.05) the neutral-detergent fiber intake and apparent digestibility of crude protein in the sheep's diet. Feeding time was increased (P<0.05) by increasing the supply of WV to the sheep; pH values decreased (P<0.05), and ruminal ammonia nitrogen increased (P<0.05) with an increasing dose of WV. The increase in the WV supplied did not influence the water absorbed and nitrogen retained by the lambs (P>0.05). The supply of WV to lambs altered the concentrations of total protein, globulin, urea, and gamma-glutamyltransferase (P<0.05). It may be advisable to offer up to 40 mL d−1 of WV to sheep fed high-concentrate diets.
- Article
(439 KB) - Full-text XML
- BibTeX
- EndNote
The confinement of sheep improves the weight gain and carcass traits of the animals compared to pasture-raised animals (Jiménez et al., 2019). However, feeding ruminants with a high level of grain can increase the production of ruminal fermentation gases and the levels of nitrogen compounds in the excreta (Wang et al., 2018). In addition, metabolic diseases such as acidosis and tympanism limit the performance of confined animals (Montano et al., 2015). In this context, nutritional additives can be used in high-grain diets to reduce negative environmental and animal impacts (Ahmed et al., 2024).
With the European market being closed to animal products from herds fed ionophore additives, there is an increasing interest in natural alternatives that can deliver similar effects to those of ionophores. Essential oils and tannins have been consolidated as alternatives to ionophore antibiotics (Orzuna-Orzuna et al., 2021; Torres et al., 2021; Yanza et al., 2021). However, the evaluation and testing of other sources of bioactive compounds are necessary. Wood vinegar (WV) has been attracting research interest in the areas of agriculture and livestock (Mhamdi, 2023; Pimenta et al., 2018). WV is a liquid byproduct of the controlled burning of lignocellulosic residues and is rich in acetic, phenolic, and carboxylic acids that have bactericidal activity against gram-positive and some gram-negative strains (Araújo et al., 2018; Jankowsky et al., 2018). The bactericidal and/or bacteriostatic action of WV seems to be associated with a change in the pH of the bacterial protoplasm (Araújo et al., 2018), similarly to that of ionophores. WV was inefficient in reducing CH4 (O'Reilly et al., 2021) but decreased the in vitro degradabilities of dry and organic matter and protozoan populations (Qomariyah et al., 2021). When tested in vivo in cattle, WV (3 % and 6 % of the total diet) reduced dry-matter intake and plasma glucose but increased daily weight gain (at the 3 % level), intramuscular fat, and meat tenderness (Kook and Kim, 2003).
WV contains several substances with antimicrobial activity, especially phenols. It is presented as an interesting alternative to synthetic microbials due to its characterization as a natural product with a beneficial effect without any commercial use restriction. Therefore, the use of WV in livestock should be studied further, mainly due to its widely described antibacterial, antifungal, and anti-inflammatory activity effects (Gama et al., 2024; Yıldızlı et al., 2024). We hypothesize that increasing doses of WV supplementation in the diet will positively affect nutrient utilization, rumen fermentation, and physiological responses in sheep fed high-concentrate diets.
All experimental procedures were performed according to the national animal welfare standards following Opinion 33/2021 of the Ethics Committee on Animal Use (CEUA) of the Universidade Federal Rural do Semi-Árido (UFERSA).
2.1 Animals, treatments, and design
The experiment was conducted at UFERSA, Mossoró, Rio Grande do Norte, Brazil (5°11′17′′ S, 37°20′39′′ W). The climate of this region is classified as “BSwh” according to the Köppen–Geiger classification, indicating a hot, semi-arid steppe climate characterized by dry winters and an average annual temperature exceeding 18 °C. The average temperature during the experiment was 27.8 °C, and average relative air humidity was 65.4 %.
The WV was purified through vacuum bi-distillation of the crude wood vinegar. The crude extract was obtained from the carbonization of the wood of a hybrid of Eucalyptus urophylla and Eucalyptus grandis (Eucalyptus urograndis – clone GG100). The production and purification procedures and the chemical composition of the wood were described in detail by Pimenta et al. (2023).
We used five castrated male sheep, Dorper × Santa Inês, with an average age of 36 months and an average body weight (BW) of 59.34±5.73 kg. Before the experiment, the animals were treated with Ripercol® L (Zoetis, USA) against endoparasites and ectoparasites and housed in metabolism cages with a feeder, a drinker, and devices for collecting feces and urine separately. The metabolism cages were installed in a shed covered with clay tile, with a ceiling height of 3.5 m and a concrete floor, and were provided with artificial lighting at night.
Before the experimental period, the animals were adapted to the high-concentrate diet by gradually increasing the proportion of concentrate in the total diet by 20 % every 8 d for 24 d, starting with a roughage : concentrate ratio of 80:20 until reaching a ratio of 20:80. The experiment lasted 85 d and was divided into five periods of 17 d, of which 12 d were for animal adaptation and 5 d were for data collection. The experiment used a Latin-square design, and the treatments consisted of doses of 0, 10, 20, 30, and 40 mL d−1 of WV.
The concentrate consisted of crushed corn grain, soybean meal, wheat bran, mineral salt, calcitic limestone, and sea salt (NaCl) (Table 1). The rations were formulated to meet the maintenance requirements of 60 kg BW male sheep (1.05 kg d−1 of dry matter, 137.00 g d−1 of crude protein (CP), and 2.02 Mcal d−1 of metabolizable energy), as calculated based on the NRC (2007) guidelines. For the roughage, Tifton 85 hay was purchased in bales of 15–18 kg, crushed in a forage machine, adjusted to produce particles of 30 mm, placed in plastic bags, and stored in a dry location. The daily food supply was divided into two meals as fully mixed feed at 08:00 and 16:00 LT (UTC−3). A 10 % surplus of the previous day's supply was recommended for voluntary intake.
The WV was administered orally using a 50 cc automatic vaccinator with an oral dosing nozzle (model 91, Walmur®, Brazil) to ensure the daily supply of the recommended levels of WV. The total dosage of WV was supplied twice, with half the dose being provided at 08:00 LT and the other half being provided at 16:00 LT, immediately after feeding. Animals that did not receive WV were given 15 mL of water as a placebo solution.
Table 1Percentage and chemical composition of the concentrate and chemical composition of the forage and diet used in the experimental diet.
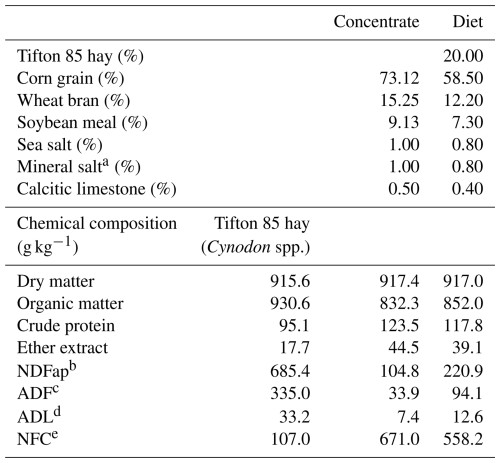
a Guaranty levels: calcium – 120 g, phosphorus – 87 g, sodium – 147 g, sulfur – 18 g, cobalt – 40 mg, copper – 590 mg, iodine – 80 mg, chrome – 20 mg, manganese – 1300 mg, selenium – 15 mg, zinc – 3800 mg, iron – 1800 mg, molybdenum – 10 mg, fluorine (max) – 870 mg. b Neutral-detergent fiber corrected for ash and protein. c Acid detergent fiber. d Acid detergent lignin. e Non-fibrous carbohydrates.
Samples of the provisioned diet, leftovers, feces, and urine were collected during the 5 d of data collection to determine the intake of nutrients, apparent digestibility, and nitrogen balance. Blood samples were taken on the fourth day, and ruminal fluid was collected on the fifth day to determine the metabolic parameters.
2.2 Nutrient intake and digestibility
The amounts of supplied diet and leftovers were recorded daily to estimate intake. Nutrient intake was calculated as the difference in chemical composition between the samples of feed supplied and the leftovers collected, which were collected, stored in plastic bags, and frozen at −15 °C until analysis. The apparent digestibility of nutrients was determined using the total feces collection method. The feces produced by each animal were collected in plastic trays adapted to the metabolism cage. The contents of the feces collection trays were weighed every 24 h at 07:00 LT, followed by homogenization and sampling of 10 % of the total weight of the fecal sample. The samples were sprayed with a sulfuric-acid solution (1:1), placed in plastic bags, and stored at −15 °C until analysis.
2.3 Analysis of the total phenol content of the wood vinegar
The total phenols in the WV were determined according to the methodology of Meda et al. (2005) using the Folin–Ciocalteau reagent. From the WV, 0.5 mL aliquots of the WV (final concentration of 20 ppm) were separately placed into test tubes; 2.5 mL of Folin–Ciocalteau reagent (0.2 N) was added to each tube; and, after 5 min, 2 mL of sodium carbonate (75 g L−1) was added. The tubes were under light for 2 h. Absorbance was measured using a methanol blank in spectrophotometry (model UV-340G, Gehaka®, Brazil) at 760 nm. The results were extrapolated in a calibration curve obtained with gallic-acid standard (20–200 ppm) and expressed in grams of gallic-acid equivalent (GAE) per 100 mL of extract. For an advanced analysis of the bi-distilled WV, see Pimenta et al. (2018).
2.4 Chemical composition analysis
The samples of diet, leftovers, and feces were thawed for 24 h in a refrigerator (4 °C), homogenized, and pre-dried in forced air ventilation ovens (model TE-394/2-MP, Tecnal®, Brazil) at 55 °C for 72 h. After pre-drying, the material was ground in a knife mill (model R-TE-680, Tecnal®, Brazil) with a 1 mm sieve and placed in hermetically sealed containers for further chemical composition analysis.
The samples were analyzed for their chemical constituents using the methods described in AOAC (2019) for dry matter (DM) (method no. 934.01); ash (MM) (method no. 942.05); CP (method no. 981.10); ether extract (EE) (method no. 920.39); and neutral-detergent-insoluble fiber (NDF), corrected for ash and protein, and acid detergent fiber according to the methodology described by Van Soest et al. (1991), with adaptations by Mertens (2002) and Licitra et al. (1996). Non-fibrous carbohydrates (NFCs) were calculated according to Detmann and Valadares Filho (2010). The diets' total digestible nutrient contents (TDN) were estimated according to the equation described by Weiss et al. (1992).
2.5 Ingestive behavior
Ingestive behavior was analyzed to determine the behavioral variables (idleness, ruminating, eating) on the first day of each collection period. The lambs were observed by a trained evaluator for 24 h (starting at 06:00 LT and ending at 05:55 LT the next day) by instantaneous scanning at intervals of 5 min, and the behavior of the animal at each time point was recorded according to the methodology described by Eustáquio Filho et al. (2016). The number of chews per ruminated bolus (no. bolus−1) and the time spent ruminating each bolus (s bolus−1), measured by using a digital stopwatch, were determined through direct observation simultaneously with the observations of the behavioral variables. Observations were made of three rumen boluses per animal during three different periods of the day (10:00–12:00, 14:00–16:00, and 18:00–20:00 LT). The feed and rumination efficiencies in DM (g h−1) and neutral-detergent-insoluble fiber (g h−1) were calculated by dividing the intake by the total time spent in feeding and/or ruminating during 24 h, respectively (Bürger et al., 2000).
2.6 Collection of ruminal fluid, pH, and ammonia nitrogen
Ruminal fluid was collected from each animal on the fifth data collection day to determine pH and ammonia nitrogen (N–NH3) levels. Approximately 100 mL of ruminal fluid was obtained with an esophageal catheter 0, 2, 4, and 6 h after the morning meal. The esophageal probe was coupled to a vacuum pump (model 131, Prismatec®, Brazil). The material sucked by the probe was filtered through double cotton gauze. After filtration, the pH was immediately determined using a digital potentiometer (model PG1800, Gehaka®, Brazil). Aliquots of 50 mL of fluid were stored in 80 mL polyethylene jars containing 1 mL of sulfuric acid (1:1) and were frozen at −15 °C for subsequent determination of the concentration of N–NH3 by the methodology described by Detmann et al. (2012).
2.7 Blood collection and serum biochemistry
Blood samples were collected from each animal by means of jugular-vein puncture on the fourth data collection day of each period, 4 h after the morning feeding, with Vacutainer tubes. The tubes for leukocyte count contained the anticoagulant ethylenediaminetetraacetic acid; the serum biochemistry tubes had no anticoagulant.
The techniques used in the blood count followed the recommendations of Jain (1993). Turk solution (1:20 dilution) was used for counting leukocytes with a hemocytometer. The samples without anticoagulant were centrifuged (model SH120, Global®, Brazil) at 5000 rpm for 15 min, stored in Eppendorf mini-tubes, and frozen at −15 °C. Subsequently, the mini-tubes were thawed at room temperature and analyzed to determine the concentrations of cholesterol, glucose, albumin, globulin, urea, creatinine, total proteins, aspartate transaminase (AST), gamma-glutamyltransaminase (GGT), and triglycerides by using commercial kits (Labtest®, Brazil), with the aid of a semi-automatic biochemical analyzer (model LB-B200, Bioplus, Brazil), as indicated by the manufacturer.
2.8 Water balance
The total water intake via drink and food was determined during the 5 d of data collection of each period. Water was supplied in 15 L polyethylene buckets offered in the metabolism cage. Daily water intake was calculated as the difference between the weight of the water supplied and the weight of the remaining water. Water losses by evaporation were determined by weighing two buckets of water strategically placed in the installation to be inaccessible to the sheep. Evaporation data were used to correct water intake.
Dietary water intake was estimated by determining the moisture content of the food and leftovers. The total water excreted was estimated by summing the volume of water excreted in feces and urine. Each animal's urine was collected in plastic trays attached to the metabolism cages during the entire collection period. To prevent nitrogen evaporation, 20 mL of sulfuric acid (1:1) was added to the trays. All urine was weighed daily, and a sample of 10 % of the total volume was collected, filtered in gauze, packaged in bottles, and frozen at −10 °C until analysis. A urine aliquot was used to determine total nitrogen (AOAC, 2019; method no. 981.10). Absorbed nitrogen (g d−1) was obtained based on the difference between ingested nitrogen and excreted nitrogen, while nitrogen retention was obtained based on consumed nitrogen (g d−1) minus excreted nitrogen minus nitrogen in urine (g d−1).
2.9 Nitrogen balance
The animals' urine was collected in plastic trays attached to the metabolism cages during the entire collection period. To prevent nitrogen evaporation, 20 mL of sulfuric acid (1:1) was added to the trays. All urine was weighed daily, and a sample of 10 % of the total volume was collected, filtered in gauze, packaged in bottles, and frozen at −10 °C until analysis. A urine aliquot was used to determine total nitrogen (AOAC, 2019; method no. 981.10). Absorbed nitrogen (g d−1) was obtained based on the difference between ingested and excreted nitrogen in feces, while nitrogen retention was obtained based on consumed nitrogen (g d−1) minus nitrogen excreted in feces minus nitrogen excreted in urine (g d−1).
2.10 Statistics
The data were subjected to an analysis of variance, and the treatment effect unfolded in its linear and quadratic components through polynomial orthogonal contrasts. The dependent variables were analyzed with a 5×5 Latin-square design using the PROC MIXED feature in the SAS On Demand for Academics software as follows:
where Yijk is a continuous dependent response variable, μijk is the overall average, Ti is the fixed effect of treatment (i=0, 10, 20, 30, or 40 mL), Pj is the effect of the period (j=1–5), Ak is the random effect of the animal within the treatment (k=1–5), and εijk is the unidentifiable random error.
When treatment effects were significant, the control diet (0 mL) was compared to the diets with WV by the Dunnett test. A level of 5 % probability for type-I errors was adopted for all procedures.
The WV tested in this study had 360.8 mg of total phenols (gallic-acid equivalent per 100 mL). Thus, the animals received 36, 72, 108, and 144 mg d−1 of total phenols in the 10, 20, 30, and 40 mL d−1 treatments, respectively.
Increasing the WV dose did not influence (P>0.05) the intake of DM, organic matter (OM), CP, and NFCs and the total digestible nutrients of sheep. However, increasing the dose of WV increased (P<0.05) the intake of NDF (Table 2).
Table 2Intake and apparent digestibility of nutrients from the diet of sheep fed high-concentrate diets and increasing levels of wood vinegar (WV).
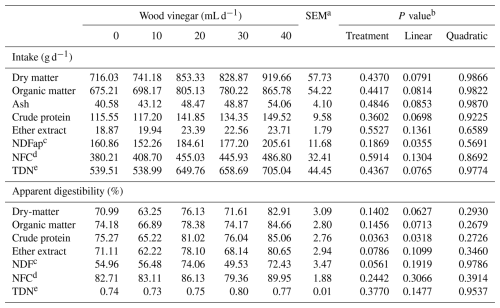
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts. c Neutral-detergent fiber corrected for ash and protein. d Non-fibrous carbohydrates. e Total digestible nutrients.
Increasing the WV dose did not influence (P>0.05) the apparent digestibilities of DM, OM, NDF, and NFCs in the diet. However, an increase in the apparent digestibility of CP was observed with an increase in WV dose. Intake time increased with an increasing WV dose. However, WV dose did not affect rumination time, total chewing time, number of ruminated boluses, or rumination efficiency (Table 3).
Table 3Ingestive behavior of sheep fed high-concentrate diets and increasing levels of wood vinegar (WV).
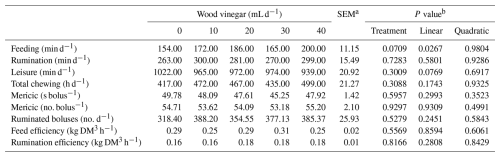
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts. c Dry matter.
Increasing the WV dose did not influence (P<0.05) the amount of water consumed by the animals. There was no effect (P<0.05) of WV dose on the amount of water absorbed or excreted (Table 4).
Table 4Water balance of sheep fed high-concentrate diets and increasing levels of wood vinegar (WV).
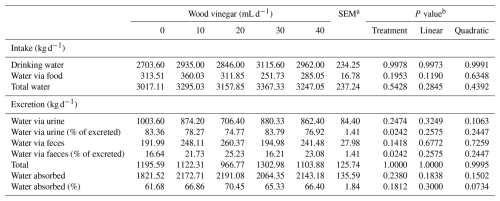
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts.
An increased WV dose did not influence (P>0.05) the amounts of nitrogen consumed, excreted, absorbed, and retained by the sheep (Table 5).
Table 5Nitrogen balance of sheep fed high-concentrate diets and increasing levels of wood vinegar (WV).
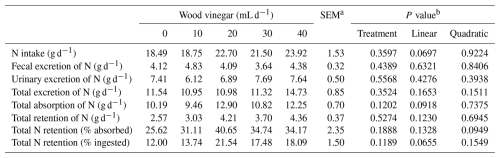
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts.
There was a linear decrease (P<0.05) in ruminal pH and a linear increase (P<0.05) in ruminal N–NH3 with increasing WV dose (Table 6). There was an increase (P<0.05) in ruminal pH and N–NH3 as a function of post-meal collection time (Table 7).
Table 6Sheep ruminal parameters as a function of increasing levels of wood vinegar (WV).
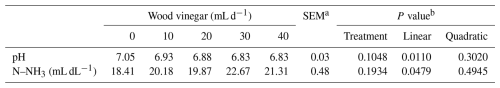
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts.
Table 7Ruminal parameters of sheep as a function of sample collection time (h) after the morning meal.
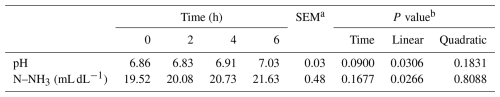
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts.
Table 8Serum biochemistry of sheep fed high-concentrate diets and increasing levels of wood vinegar (WV).
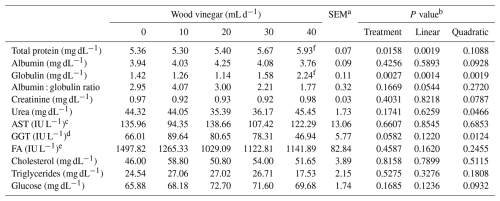
a SEM denotes standard error of the mean. b P value for linear and quadratic order contrasts. c Aspartate aminotransferase. d Gamma-glutamyltransferase. e Alkaline phosphatase. f Means that differ from the control (0 mL d−1 of WV) according to the Dunnett's test at the level of 5 % probability.
Increasing the WV dose increased (P<0.05) the serum protein levels and influenced (P<0.05) the levels of globulin and urea in a negative quadratic manner. There was a positive quadratic response in GGT levels to an increasing WV dose (Table 8). The dose of WV did not influence glucose, cholesterol, or triglyceride levels (P>0.05).
WV is rich in phenolic compounds such as guaiacol, phenol, cresol, and furfural (Pimenta et al., 2018). These antimicrobial compounds can function as a ruminal fermentation modulator and animal growth promoter (Gama et al., 2024). Additionally, WV is an acidic liquid (pH 2.83). Its supply to animals may stimulate adaptive mechanisms to buffer ruminal pH (González et al., 2012). In this context, we suggest that the observed increase in NDF intake, combined with the increase in WV dose, is associated with the sheep's attempt to regulate ruminal pH since WV may have acidified the ruminal fluid (Moya et al., 2011). Ruminants have chemoreceptors that can detect changes in the chemical characteristics of their ruminal content and stimulate feedback loops, such as increased intake of NDF to favor chewing and salivation, which help control ruminal pH (Clauss and Hummel, 2017). The amount of phenolic compounds consumed by sheep (0.05 0.08 g kg−1 DM; 0.13 and 0.16 g kg−1 DM) was low according to the meta-analysis of Al Rharad et al. (2025).
Phenolic compounds can alter the ingestive behavior of ruminants (Santos et al., 2021). The increase in food intake time with an increased WV dose can be attributed to the greater intake of roughage – the primary source of NDF – by the animals (Fimbres et al., 2002). Roughage requires a longer chewing period than the bran concentrate to reduce particle size (Mendes et al., 2020). On the other hand, we observed no change in rumination time or the amount of bolus ruminated by the animals, possibly due to there being the same roughage : concentrate ratio in all treatments (Eustáquio Filho et al., 2016). Ahmed et al. (2021) reported an increase in the feeding time of sheep subjected to phytogenic substances in the diet.
Associated with the increased NDF intake is the possibility that there was a reduction in the rate of disappearance of ruminal DM due to the increase in NDF in the ruminal content itself (Goulart et al., 2020). In this context, this reduction in the ruminal DM passage rate may be associated with increased ruminal starch degradation – the primary component of the sheep's diet – and increased energy intake within the rumen (Cui et al., 2019), supporting greater bacterial growth and ruminal microbial protein synthesis (Guo et al., 2021; Zhang et al., 2024) with an increased WV dose. Therefore, the increase in the digestibility of CP with an increased WV dose may be associated with increased synthesis of ruminal microbial protein (Putri et al., 2021), which has greater digestibility and biological value than food protein (Santos et al., 2021).
Increased doses of WV (an acidic liquid) associated with (probable) greater microbial growth – with greater production of organic acids by bacteria – possibly explain the decrease in ruminal pH (Carlis et al., 2021) that we observed in sheep receiving higher doses of WV. In this context, the increase in ruminal N–NH3 with an increased WV dose may also be associated with greater microbial growth and its positive effect on the degradation of potentially fermentable OM, mainly degradable protein in sheep's rumen (Shen et al., 2023).
Despite the increase in the apparent digestibility of CP and ruminal N–NH3, we observed no effect of WV dose on the excretion, absorption, and retention of nitrogen. We did not expect these results since the effect of phenolic compounds as modulators of nitrogen absorption and retention in the body of animals is well documented (Ahmed et al., 2024). We suggest that the type of diet used (high-concentrate) in all treatments may have interfered with the mechanisms inherent to the sheep's nitrogen balance, such as nitrogen recycling, serum protein production, and liver transaminases (Abdoun et al., 2006; Bach et al., 2005).
The increase in the digestibility of CP, with a larger amino acid pool absorbed in the small intestine of sheep, explains the higher levels of total protein in the serum of sheep that received higher doses of WV (Buryakov et al., 2022). Additionally, the increasing availability of N–NH3 for absorption by the ruminal epithelium, with increased doses of WV, resulted in greater substrate for hepatic-urea synthesis and increased serum urea (Hanigan et al., 2018). On the other hand, the quadratic serum urea response may be associated with the increased hepatic transamination (see quadratic GGT elevation) associated with serum protein synthesis (see quadratic globulin elevation) (Hristov et al., 2019).
The supply of up to 40 mL d−1 of WV increases NDF intake, CP apparent digestibility, and N–NH3 in sheep rumens. We recommend offering up to 40 mL d−1 of WV to sheep fed high-concentrate diets. Studies with higher doses of WV are needed. Future studies may focus on evaluating the impact of using WV on different types of diets, growth performance, and the meat or milk quality of ruminants.
No data sets were used in this article.
V. L. L. Melo, P. O. Lima, S. S. Firmino, P. P. N. Oliveira, A. S. Pimenta, M. F. Macedo, J. G. L. Almeida, A. V. A. F. Amâncio, and M. R. T. Dantas: writing (original draft, review and editing), methodology, conceptualization. D. M. Lima Júnior and T. L. A. C. Araújo: writing (original draft, review and editing), project administration, funding acquisition, conceptualization.
The contact author has declared that none of the authors has any competing interests.
All experimental procedures were performed according to the national animal welfare standards following Opinion 33/2021 of the Ethics Committee on Animal Use (CEUA) of the Universidade Federal Rural do Semi-Árido (UFERSA).
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (Doctor's fellowship grant) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq.
This paper was edited by Manfred Mielenz and reviewed by Hande Akbağ and one anonymous referee.
Abdoun, K., Stumpff, F., and Martens, H.: Ammonia and urea transport across the rumen epithelium: a review, Anim. Health Res. Rev., 7, 43–59, https://doi.org/10.1017/S1466252307001156, 2006.
Ahmed, E., Batbekh, B., Fukuma, N., Kand, D., Hanada, M., and Nishida, T.: A garlic and citrus extract: Impacts on behavior, feed intake, rumen fermentation, and digestibility in sheep, Anim. Feed Sci. Technol., 278, 115007, https://doi.org/10.1016/j.anifeedsci.2021.115007, 2021.
Ahmed, M. G., Elwakeel, E. A., El-Zarkouny, S. Z., and Al-Sagheer, A. A.: Environmental impact of phytobiotic additives on greenhouse gas emission reduction, rumen fermentation manipulation, and performance in ruminants: an updated review, Environment. Sci. Pollut. Res., 31, 37943–37962, https://doi.org/10.1007/S11356-024-33664-5, 2024.
Al Rharad, A., El Aayadi, S., Avril, C., Souradjou, A., Sow, F., Camara, Y., Hornick, J.-L., and Boukrouh, S. Meta-Analysis of Dietary Tannins in Small Ruminant Diets: Effects on Growth Performance, Serum Metabolites, Antioxidant Status, Ruminal Fermentation, Meat Quality, and Fatty Acid Profile, Animals, 15, 596, https://doi.org/10.3390/ani15040596, 2025.
AOAC: Official Methods of Analysis, 21st edn., edited by: Latimer Jr., G., Association of Official Agricultural Chemists, Gaithersburg, ISBN 978-0935584899, 2019.
Araújo, E. de S., Pimenta, A. S., Feijó, F. M. C., Castro, R. V. O., Fasciotti, M., Monteiro, T. V. C., and de Lima, K. M. G.: Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora, J. Appl. Microbiol., 124, 85–96, https://doi.org/10.1111/jam.13626, 2018.
Bach, A., Calsamiglia, S., and Stern, M. D.: Nitrogen Metabolism in the Rumen, J. Dairy Sci., 88, E9–E21, https://doi.org/10.3168/jds.S0022-0302(05)73133-7, 2005.
Bürger, P. J., Pereira, J. C., Queiroz, A. C. de, Coelho da Silva, J. F., Valadares Filho, S. de C., Cecon, P. R., and Casali, A. D. P.: Comportamento ingestivo em bezerros holandeses alimentados com dietas contendo diferentes níveis de concentrado, R. Bras. Zootec., 29, 236–242, https://doi.org/10.1590/S1516-35982000000100031, 2000.
Buryakov, N., Aleshin, D., Buryakova, M., Zaikina, A., Nasr, M., Nassan, M., and Fathala, M.: Productive Performance and Blood Biochemical Parameters of Dairy Cows Fed Different Levels of High-Protein Concentrate, Front. Vet. Sci., 9, 852240, https://doi.org/10.3389/fvets.2022.852240, 2022.
Carlis, M. S. de P., Sturion, T. U., Silva, A. L. A. da, Eckermann, N. R., Polizel, D. M., Assis, R. G., Souza, T. T., Dias Junior, P. C. G., Vicente, A. C. S., Santos, I. J., Comelli, J. H., Baiva, J. S., Pires, A. V., and Ferreira, E. M.: Whole corn grain-based diet and levels of physically effective neutral detergent fiber from forage (pefNDF) for feedlot lambs: Digestibility, ruminal fermentation, nitrogen balance and ruminal pH, Small Rum. Res., 205, 106567, https://doi.org/10.1016/j.smallrumres.2021.106567, 2021.
Clauss, M. and Hummel, J.: Physiological adaptations of ruminants and their potential relevance for production systems, R. Bras. Zootec., 46, 606–613, https://doi.org/10.1590/s1806-92902017000700008, 2017.
Cui, K., Qi, M., Wang, S., Diao, Q., and Zhang, N.: Dietary energy and protein levels influenced the growth performance, ruminal morphology and fermentation and microbial diversity of lambs, Sci. Rep., 9, 1–10, https://doi.org/10.1038/s41598-019-53279-y, 2019.
Detmann, E. and Valadares Filho, S. C.: On the estimation of non-fibrous carbohydrates in feeds and diets, Arq. Bras. Med. Vet. Zootec., 62, 980–984, https://doi.org/10.1590/S0102-09352010000400030, 2010.
Detmann, E., Souza, M. A. de, Valadares Filho, S. de C., Queiroz, A. C. de, Berchielli, T. T., Saliba, E. de O. S., Cabral, L. da S., Pina, D. dos S., Ladeira, M. M., and Azevedo, J. A. G.: Métodos para análise de alimentos, Visconde do Rio Branco, Suprema, 214, ISBN 9788581790206, 2012.
Eustáquio Filho, A., Carvalho, G. G. P., Pires, A. J. V., Silva, R. R., Santos, P. E. F., Murta, R. M., Pereira, F. M., Carvalho, B. M. A., Maranhão, C. M. A., Rufino, L. M. A., Santos, S. A., and Pina, D. S.: Intake and ingestive behavior in lambs fed low-digestibility forages, Trop. Anim. Health Prod., 48, 1315–1321, https://doi.org/10.1007/s11250-016-1090-x, 2016.
Fimbres, H., Kawas, J. R., Hernández-Vidal, G., Picón-Rubio, J. F., and Lu, C. D.: Nutrient intake, digestibility, mastication and ruminal fermentation of lambs fed finishing ration with various forage levels, Small Rum. Res., 43, 275–281, https://doi.org/10.1016/S0921-4488(02)00013-5, 2002.
Gama, G. S. P., Pimenta, A. S., Feijó, F. M. C., de Azevedo, T. K. B., de Melo, R. R., and de Andrade, G. S.: The Potential of Wood Vinegar to Replace Antimicrobials Used in Animal Husbandry – A Review, Animals, 14, 381, https://doi.org/10.3390/ANI14030381, 2024.
González, L. A., Manteca, X., Calsamiglia, S., Schwartzkopf-Genswein, K. S., and Ferret, A.: Ruminal acidosis in feedlot cattle: Interplay between feed ingredients, rumen function and feeding behavior (a review), Anim. Feed Sci. Technol., 172, 66–79, https://doi.org/10.1016/j.anifeedsci.2011.12.009, 2012.
Goulart, R. S., Vieira, R. A. M., Daniel, J. L. P., Amaral, R. C., Santos, V. P., Toledo Filho, S. G., Cabezas-Garcia, E. H., Tedeschi, L. O., and Nussio, L. G.: Effects of source and concentration of neutral detergent fiber from roughage in beef cattle diets on feed intake, ingestive behavior, and ruminal kinetics, J. Anim. Sci., 98, 1–15, https://doi.org/10.1093/JAS/SKAA107, 2020.
Guo, T., Wang, Z. L., Guo, L., Li, F., and Li, F.: Effects of supplementation of nonforage fiber source in diets with different starch levels on growth performance, rumen fermentation, nutrient digestion, and microbial flora of Hu lambs, Transl. Anim. Sci., 5, txab065, https://doi.org/10.1093/tas/txab065, 2021.
Hanigan, M. D., White, R. R., Apelo, S. I. A., Aguilar, M., Estes, K. A., and Myers, A.: Predicting post-absorptive protein and amino acid metabolism, R. Bras. Zootec., 47, e20160417, https://doi.org/10.1590/RBZ4720160417, 2018.
Hristov, A. N., Bannink, A., Crompton, L. A., Huhtanen, P., Kreuzer, M., McGee, M., Nozière, P., Reynolds, C. K., Bayat, A. R., Yáñez-Ruiz, D. R., Dijkstra, J., Kebreab, E., Schwarm, A., Shingfield, K. J., and Yu, Z.: Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques, J. Dairy Sci., 102, 5811–5852, https://doi.org/10.3168/jds.2018-15829, 2019.
Jain, N. C.: Essentials of veterinary hematology, Lea & Febiger, Philadelphia, 417 pp., ISBN 9780812114379, 1993.
Jankowsky, L., Lira, S. P. de, Tanaka, F. A. O., Jankowsky, I. P., and Brito, J. O.: Antimicrobial Activity of the Methanolic Fraction of Bamboo Pyroligneous Liquor, J. Pharm. Pharmacol., 6, https://doi.org/10.17265/2328-2150/2018.10.005, 2018.
Jiménez, L. E. R., Naranjo, A., Hernandez, J. C. A., Ovalos, J. O., Ortega, O. C., and Ronquillo, M. G.: A meta-analysis on the effect of the feeding type and production system on the carcase quality of lambs, Ital. J. Anim. Sci., 18, 423–434, https://doi.org/10.1080/1828051X.2018.1532327, 2019.
Kook, K. and Kim, K. H.: The Effects of Supplemental Levels of Bamboo Vinegar on Growth Performance, Serum Profile and Meat Quality in Fattening Hanwoo Cow, J. Anim. Sci. Technol., 45, 57–68, https://doi.org/10.5187/JAST.2003.45.1.057, 2003.
Licitra, G., Hernandez, T. M., and Van Soest, P. J.: Standardization of procedures for nitrogen fractionation of ruminant feeds, Anim. Feed Sci. Technol., 57, 347–358, https://doi.org/10.1016/0377-8401(95)00837-3, 1996.
Meda, A., Lamien, C. E., Romito, M., Millogo, J., and Nacoulma, O. G.: Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity, Food Chem., 91, 571–577, https://doi.org/10.1016/j.foodchem.2004.10.006, 2005.
Mendes, J. A. C., Maia Parente, M. O., Nunes Parente, H., Zanine, A. M., Ferreira, D. J., Moreira Filho, M. A., Leme Da Cunha, I. A., Ladim, A. V., and Sousa Da Rocha, K.: Performance, ingestive behavior and cost of production of finishing lambs fed non-forage diets, Biol. Rhythm Res., 51, 460–470, https://doi.org/10.1080/09291016.2018.1535540, 2020.
Mertens, D. R.: Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study., J AOAC Int., 85, 1217–40, 2002.
Mhamdi, R.: Evaluating the evolution and impact of wood vinegar research: A bibliometric study, J. Anal. Appl. Pyrolysis, 175, 106190, https://doi.org/10.1016/J.JAAP.2023.106190, 2023.
Montano, M. F., Manriquez, O. M., Salinas-Chavira, J., Torrentera, N., and Zinn, R. A.: Effects of monensin and virginiamycin supplementation in finishing diets with distiller dried grains plus solubles on growth performance and digestive function of steers, J. Appl. Anim. Res., 43, 417–425, https://doi.org/10.1080/09712119.2014.978785, 2015.
Moya, D., Mazzenga, A., Holtshausen, L., Cozzi, G., González, L. A., Calsamiglia, S., Gibb, D. G., McAllister, T. A., Beauchemin, K. A., and Schwartzkopf-Genswein, K.: Feeding behavior and ruminal acidosis in beef cattle offered a total mixed ration or dietary components separately, J. Anim. Sci., 89, 520–530, https://doi.org/10.2527/JAS.2010-3045, 2011.
NRC: Nutrient Requirement of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids, The National Academies Press, Washington, DC, 362 pp., ISBN 978-0309102131, 2007.
O'Reilly, G. C., Huo, Y., Meale, S. J., and Chaves, A. V.: Dose response of biochar and wood vinegar on in vitro batch culture ruminal fermentation using contrasting feed substrates, Transl. Anim. Sci., 5, txab107, https://doi.org/10.1093/TAS/TXAB107, 2021.
Orzuna-Orzuna, J., Dorantes-Iturbide, G., Lara-Bueno, A., Mendoza-Martínez, G., Miranda-Romero, L., and Hernández-García, P.: Effects of Dietary Tannins' Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis, Sustainability, 13, 7410, https://doi.org/10.3390/su13137410, 2021.
Pimenta, A., Fasciotti, M., Monteiro, T., and Lima, K.: Chemical Composition of Pyroligneous Acid Obtained from Eucalyptus GG100 Clone, Molecules, 23, 426, https://doi.org/10.3390/molecules23020426, 2018.
Pimenta, A. S., Gama, G. S. P., Feijó, F. M. C., Braga, R. M., de Azevedo, T. K. B., de Melo, R. R., de Oliveira Miranda, N., and de Andrade, G. S.: Wood Vinegar from Slow Pyrolysis of Eucalyptus Wood: Assessment of Removing Contaminants by Sequential Vacuum Distillation, Forests, 14, 2414, https://doi.org/10.3390/f14122414, 2023.
Putri, E. M., Zain, M., Warly, L., and Hermon, H.: Effects of rumen-degradable-to-undegradable protein ratio in ruminant diet on in vitro digestibility, rumen fermentation, and microbial protein synthesis, Vet. World, 14, 640–648, https://doi.org/10.14202/vetworld.2021.640-648, 2021.
Qomariyah, N., Retnani, Y., Jayanegara, A., Wina, E., and Permana, I. G.: Gas production kinetic and organic matter digestibility in vitro of diet supplemented by biochar and liquid smoke, IOP Conf. Ser. Mater Sci. Eng., 1098, 062031, https://doi.org/10.1088/1757-899X/1098/6/062031, 2021.
Santos, J. D. C. dos, Saraiva, E. P., Gonzaga Neto, S., Saraiva, C. A. S., Pinheiro, A. da C., Fonsêca, V. de F. C., Santos, S. G. C. G. dos, Souza, C. G. de, Almeida, M. E. V., Veríssimo, T. N. S., and Morais, L. K. da C.: Feeding Behavior of Lactating Dairy Cattle Fed Sorghum-Based Diets and Increasing Levels of Tannic Acid, Agriculture, 11, 172, https://doi.org/10.3390/agriculture11020172, 2021.
Shen, J., Zheng, W., Xu, Y., and Yu, Z.: The inhibition of high ammonia to in vitro rumen fermentation is pH dependent, Front. Vet. Sci., 10, 1163021, https://doi.org/10.3389/fvets.2023.1163021, 2023.
Torres, R. N. S., Paschoaloto, J. R., Ezequiel, J. M. B., da Silva, D. A. V., and Almeida, M. T. C.: Meta-analysis of the effects of essential oil as an alternative to monensin in diets for beef cattle, The Veterinary J., 272, 105659, https://doi.org/10.1016/j.tvjl.2021.105659, 2021.
Van Soest, P. J., Robertson, J. B., and Lewis, B. A.: Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition, J. Dairy Sci., 74, 3583–3597, https://doi.org/10.3168/jds.S0022-0302(91)78551-2, 1991.
Wang, Y., Li, X., Yang, J., Tian, Z., Sun, Q., Xue, W., and Dong, H.: Mitigating Greenhouse Gas and Ammonia Emissions from Beef Cattle Feedlot Production: A System Meta-Analysis, Environ. Sci. Technol., 52, 11232–11242, https://doi.org/10.1021/acs.est.8b02475, 2018.
Weiss, W. P., Conrad, H. R., and St. Pierre, N. R.: A theoretically-based model for predicting total digestible nutrient values of forages and concentrates, Anim. Feed Sci. Technol., 39, 95–110, https://doi.org/10.1016/0377-8401(92)90034-4, 1992.
Yanza, Y. R., Fitri, A., Suwignyo, B., Elfahmi, Hidayatik, N., Kumalasari, N. R., Irawan, A., and Jayanegara, A.: The Utilisation of Tannin Extract as a Dietary Additive in Ruminant Nutrition: A Meta-Analysis, Animals, 11, 3317, https://doi.org/10.3390/ani11113317, 2021.
Yıldızlı, G., Coral, G., and Ayaz, F.: Anti-bacterial, anti-fungal, and anti-inflammatory activities of wood vinegar: a potential remedy for major plant diseases and inflammatory reactions, Biomass Convers. Biorefin., 14, 3633–3642, https://doi.org/10.1007/s13399-022-02482-5, 2024.
Zhang, Z., Wang, L., Li, Q., Li, F., Ma, Z., Li, F., Wang, Z., Chen, L., Yang, X., Wang, X., and Yang, G.: Effects of dietary forage neutral detergent fiber and rumen degradable starch ratios on chewing activity, ruminal fermentation, ruminal microbes and nutrient digestibility of Hu sheep fed a pelleted total mixed ration, J. Anim. Sci., 102, skae100, https://doi.org/10.1093/jas/skae100, 2024.




