the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Genetic polymorphism of the ovine MAP3K5 gene and its association with body size traits in Hu sheep of China
Xiaobin Yang
Weimin Wang
Deyin Zhang
Xiaolong Li
Yukun Zhang
Yuan Zhao
Liming Zhao
Jianghui Wang
Dan Xu
Jiangbo Cheng
Wenxin Li
Bubo Zhou
Changchun Lin
Xiwen Zeng
Rui Zhai
Zongwu Ma
Jia Liu
Panpan Cui
Xiaoxue Zhang
As an excellent local sheep breed in China, Hu sheep have the characteristics of producing more lambs and good motherhood. The purpose of this study was to identify the polymorphism of the mitogen-activated protein kinase 5 (MAP3K5) gene and determine whether it was associated with the body size traits (body height, body length, chest circumference, and cannon circumference) in Hu sheep. The polymorphism of MAP3K5 was identified by using PCR amplification, followed by Sanger sequencing, and KASPar (Kompetitive Allele Specific PCR) technology was used for genotyping subsequently. The results of the association analysis showed that MAP3K5 (g.205261 A > G) was significantly associated with body height at 80, 100, 140, 160, and 180 d; body length at 80 and 160 d; chest circumference at 100, 140, 160, and 180 d; and cannon circumference at 100 and 120 d, respectively. The results of qRT-PCR showed that the expression level of MAP3K5 in the heart was significantly higher (P < 0.05) than in the other 10 tissues. In summary, the MAP3K5 mutation loci may be used as a candidate molecular marker for the body size traits of Hu sheep.
- Article
(1700 KB) - Full-text XML
-
Supplement
(491 KB) - BibTeX
- EndNote
As early domesticated animals, sheep provide meat, milk, and other products for humans (Awawdeh, 2011; Li et al., 2018). Hu sheep originated in the Taihu region and is one of the local breeds in China. It has the characteristics of high reproduction rate, fast growth, and more robust environmental adaptability (H et al., 2020; Chen et al., 2020). With the development of the economy and improvement in living standards, Chinese demand for sheep is increasing year after year. Of course, the quality of the sheep is a very important issue, but we should also pay attention to the production of sheep. Breeding will play a vital role in resolving the issue of insufficient supply of sheep (H et al., 2020). Excellent livestock breeds play an essential role in livestock development (Abraham et al., 2018; Quan et al., 2020). Body size traits include body height, body length, chest circumference, and cannon circumference, etc., which are influenced by many factors and also play significant roles in production practice. There are also various methods of animal breeding; compared to traditional breeding methods, researchers indicate that molecular marker technology will help establish excellent traits. Pedersen et al. (2009) showed that marker-assisted selection is a useful selection strategy when comparing BLUP (best linear unbiased prediction), QTL (quantitative trait locus), and MAS (molecular marker-assisted selection) (Pedersen et al., 2009). Therefore, in the breeding practice, we can use molecular marker technology to select sheep with more prominent body size traits for production, to maximize the expansion of production efficiency, reduce production costs, and increase the supply of meat products.
Mitogen-activated protein kinase 5 (MAP3K5), also known as apoptosis signal-regulating kinase 1 (ASK1), is a member of the mitogen-activated protein kinase family (MAPK) (Pu et al., 2016; Takenaka et al., 2020). The MAPK family was first elucidated in 1994 and is involved in the control of the cell cycle, apoptosis, and differentiation. Kyriakis and Avruch (2012) demonstrated that the MAPK signaling pathway plays an important role in the cellular physiology of mammals (Kyriakis and Avruch, 2012). MAP3K5 plays an important role in cell response cascades caused by environmental changes. A study by Sirotkin et al. (2008) found that the transcription factor p53, which is mainly located in the nucleus, can stimulate the expression of MAP3K5, which is mainly located in the cytoplasm (Sirotkin et al., 2008). In addition, the study of mice, Hattori et al. (2009) found that apoptosis induced by tumor necrosis factor α or hydrogen peroxide was inhibited in mouse embryonic fibroblasts deficient in ASK1 (Hattori et al., 2009). Harada et al. (2006) found that inhibition of the ASK1-p38 pathway could be used to treat neurodegenerative diseases including glaucoma (Harada et al., 2006). Some researchers have shown that MAP3K5 was highly expressed in the heart of Duroc pigs, which indicated that this was related to their high metabolic capacity (Pu et al., 2016). Although there are many studies related to this gene and previous studies have shown that the MAP3K5 gene can affect physiological processes in animal cells, the influence of the MAP3K5 gene on sheep body size traits is not clear. Consequently, we explored the relationship between the single nucleotide polymorphism (SNP) of the MAP3K5 gene and the body size traits of Hu sheep in this study, which has a certain value for sheep breeding and screening of sheep with excellent body size traits.
2.1 Experimental animals and DNA extraction
The animals used in this study were farmed in Minqin Defu Agriculture Co. Ltd. (Gansu, China). All lambs were purchased from four commercial sheep farms (including Jinchang Zhongtian Sheep Industry Co. Ltd., Gansu Sanyangjinyuan Husbandry Co. Ltd., Gansu Zhongsheng Huamei Sheep Industry Development Co. Ltd., and Wuwei Pukang Breeding Co. Ltd. in Gansu Province, China). All these sheep received a standardized vaccination program before weaning.
All the lambs had a 10 d experimental preparation period, a 14 d adaptation period to the new conditions of the environment, and a 100 d experimental period. The feeding, management conditions and environment of the whole experiment were consistent; furthermore, ad libitum feeding was followed. A total of 1388 male Hu sheep were housed in a single column of 0.8 × 1 m from 56 to 180 d old. Body size traits of all lambs were recorded, which included body height, body length, chest circumference, and cannon circumference at the 80th, 100th, 120th, 140th, 160th, and 180th days before feeding in the morning. During the whole experiment process, it was ensured that one or two people in charge measured the sheep's body size trait data as much as possible, to reduce unnecessary measurement errors. From the jugular vein of all sheep, 5 mL of blood was collected when they were at 180 d, and DNA was extracted using a kit (TransGen Biotech, Beijing, China), which was dissolved in an elution buffer. And then they were dissolved in an elution buffer (10 mM tris hydrochloride and 1 mM ethylenediamine tetra acetic acid, pH 8.0). NANODROP ONE (Thermo Fisher Scientific, USA) was used to detect the concentration and purity of the obtained DNA templates, which were temporarily stored in −20 ∘C until needed.
2.2 SNP identification and genotyping
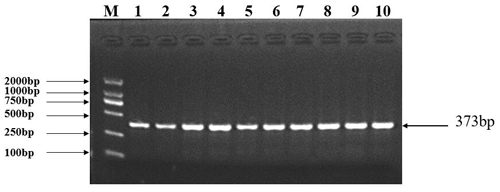
The specific PCR primers for MAP3K5 (GenBank accession NC_040259.1) were designed by Oligo 7 and Primer 5 software. The most suitable annealing temperature for the primer of the MAP3K5 gene was determined by a gradient PCR instrument. Then the PCR products were produced from amplification with mixed DNA as the template, which was sequenced to identify the SNP in MAP3K5. The specific PCR primers for MAP3K5 are shown in Table 1. The PCR product was obtained by using the mixed DNA of 10 sheep randomly selected from the experimental population as a template (Fig. 2). The PCR was performed using a mixture of a total volume of 35 µL, containing 17.5 µL Master Mix (TSINGKE Biological Technology, Beijing, China), 1.0 µL forward and reverse primers each, 1.5 µL template DNA, and 14 µL of H2O. Before that, the optimum temperature screening of the MAP3K5 gene was conducted, and the results are shown in Figs. S1a and b in the Supplement. The cycling conditions were as follows: 3 min at 94 ∘C, followed by 30 s at 94 ∘C, 30 s at 45–65 ∘C, 30 s at 72 ∘C for 35 cycles, and finally 5 min at 72 ∘C. As reported by a previous study, the SNP identified MAP3K5, and genotype-specific primers were used for competitive PCR (KASPar) (He et al., 2014). The information on the primers used in KASPar is showed in Table 1. In this study, a total of 1355 sheep were successfully genotyped.
2.3 Tissue expression analysis
Six sheep's heart, liver, spleen, lung, kidney, rumen, lymph, muscle, duodenum, tail fat, and bone tissues were randomly selected, and RNA was extracted from these tissues with Transzol up (TransGen, Beijing, China) and chloroform. A reverse transcriptase kit (Accurate Biotechnology Co., Ltd, Hunan, China) was used to reverse transcribe the RNA into cDNA and then qRT-PCR after the detection concentration and quality are qualified. Each sample will undergo four technical replicates and six biological replicates. The qRT-PCR components were as follows: 6.4 µL RNase-free water, 10 µL 2× SYBR Green PCR Master Mixture (Takara Biotechnology), 0.8 µL upstream and downstream primers, and 2.0 µL template cDNA. The apparatus of LightCycler® 480 (Roche, Basel, Sweden) was used for experimental analysis, and the reaction conditions of qRT-PCR were as follows: 94 ∘C for 5 min, followed by 34 cycles of 94 ∘C for 30 s, 57 ∘C for 30 s, 72 ∘C for 30 s, and finally 72 ∘C for 5 min (Wang et al., 2014). The data were analyzed with the 2−CT method (Livak and Schmittgen, 2001).
2.4 Statistical analysis
The genotype frequency, allele frequency, expected heterozygosity (He), expected homozygosity (Ho), allele number (Ne), polymorphism information content (PIC), and the P value of Hardy–Weinberg equilibrium (PHWE) were calculated based on previous research (Zhao et al., 2013). SPSS 24.0 software was used to analyze the relationship between different genotypes and body size traits. Body size traits based on the following general linear model, where genotypes, seasons, and batches were fixed factors (main effects), and the phenotypic data of experimental populations corresponding to different genotypes was the dependent variable. The specific model was defined as follows:
In this model, Yijk represents the phenotype of body size traits and μ is the average. Genotypei represents the effect of ith genotypes. Batchj represents the effect of jth batch (j=2, 3, 4, 5, 6, 7). Seasonk represents the effect of kth season (k=1, 2) and εijk represents random error. Duncan's test was used to analyze the association between different genotypes and phenotypic traits. P<0.05 indicated statistical significance.
3.1 Descriptive statistics of body size traits
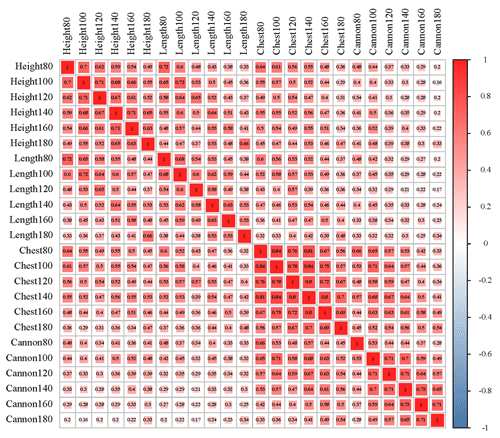
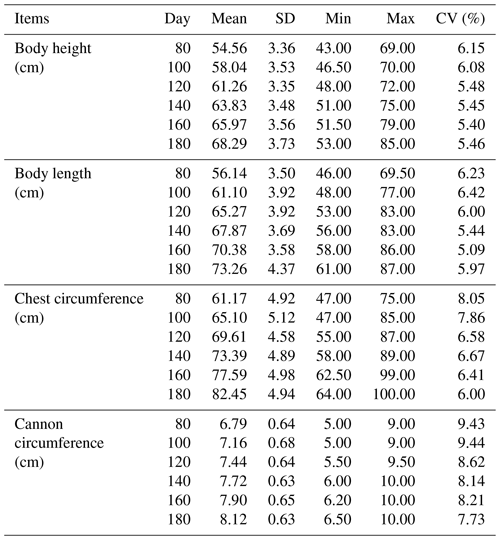
The results of descriptive statistics of body size traits in different stages are shown in Table 2. With increasing age, body size traits increased to varying degrees. The correlation analysis of body size traits is shown in Fig. 1, which suggests that the traits of body height and body length have the highest correlation at the same stage. In contrast, the correlation between body height and cannon circumference was relatively low. It was worth noting that these four traits all showed a positive correlation.
3.2 SNP of sheep MAP3K5
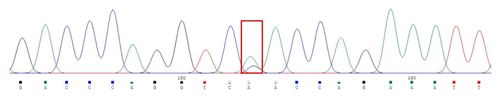
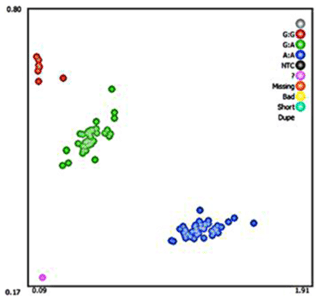
A silent mutation at g.205261 A > G was identified by sequencing in MAP3K5 (Fig. 3). The SNP g.205261 A > G in MAP3K5 was located in the intron 25. This SNP locus was typed by KASPar technology, three genotypes were identified: GG, GA, and AA (Fig. 4).
3.3 Genetic parameters of SNP
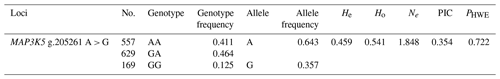
The genetic parameters of the SNP g.205261 A > G are shown in Table 3. The genotypic frequencies of AA, GA, and GG were 0.406, 0.469, and 0.125, respectively. In addition, the results showed that A was A-dominant allele, and its allele frequency was 0.640. In addition, the He, Ho, Ne, PIC, and PHWE for MAP3K5 were 0.461, 0.539, 1.855, 0.433, and 0.722, respectively.
3.4 Association analysis of body size traits at different stages
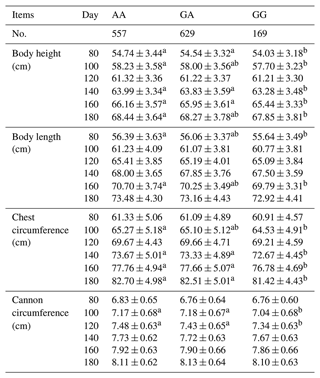
A general linear statistical model was used to estimate the association between the SNP g.205261 A > G of the MAP3K5 gene and body size traits. The results showed that the MAP3K5 gene had a certain association with different body size traits at different stages, which were shown in Table 4. Among the body height, there was a significant association on the 80th, 100th, 140th, 160th, and 180th days (P<0.05). For the body length, there was a significant association between the 80th and 160th days (P<0.05). For the chest circumference, there was a significant association on the 100th, 140th 160th, and 180th days (P<0.05). Among the cannon circumference, there was a significant association on the 100th and 120th days (P<0.05). The results showed that the body height, body length, chest circumference, and cannon circumference of AA genotype sheep were significantly higher than those of GG genotype sheep, which proved that AA genotype was the main effect on sheep body height, body length, chest circumference, and cannon circumference.
3.5 Analysis of RNA expression profiles in various tissues of sheep
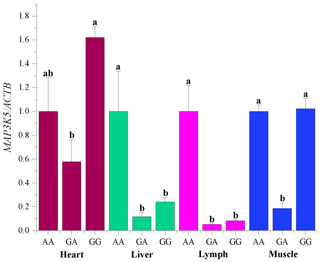
The mRNA sequences of sheep MAP3K5 g.205261 G > A (GenBank accession no. XM_042253518) were used as templates (Table 5). The RNA expression profiles showed that the MAP3K5 gene was widely expressed in these tissues, and the expression level in the heart was significantly higher than that of the other tissues (including lung, kidney, rumen, tail fat, liver, spleen, lymph, muscle, duodenum, and bone; P<0.05, Fig. 5). The expression levels of the MAP3K5 gene in different genotypes of Hu sheep are shown in Fig. 6. The results showed that there was no significant difference in the expression levels of the MAP3K5 gene in the heart and muscle tissues of Hu sheep with AA and GG genotypes, while the expression levels of the MAP3K5 gene in the liver and lymph tissues of Hu sheep with an AA genotype were significantly higher than those of Hu sheep with a GG genotype (P<0.05).
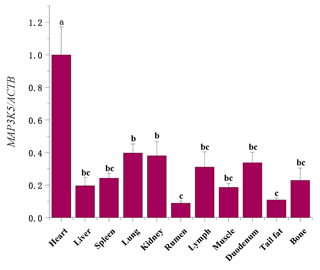
Figure 5The mRNA expression profiles of the MAP3K5 gene in various tissues of Hu sheep. Different lowercase letters indicate significant differences (P<0.05).
Livestock and poultry germplasm resources and molecular breeding technology play very important roles in animal production and breeding. Therefore, it is necessary to study the association between genes and traits (Beuzen et al., 2000; Clifford et al., 2010).
This study investigated the relationship between the MAP3K5 g.205261 A > G silencing mutation and body height, body length, chest circumference, and cannon circumference of Hu sheep. The g.205261 A > G mutation of the MAP3K5 gene was significantly associated with the body height at 80, 100, 140, 160, and 180 d; the body length at 80 and 160 d; the chest circumference at 100, 140, 160, and 180 d; and the cannon circumference at 100 and 120 d. Similar to the results of most previous studies, the silent mutation is regarded as a kind of “silence” and does not affect the traits of the organism. However, relevant new research results show that silent mutation can cause abnormal splicing cassette stability of mRNA, thereby affecting the normal expression of protein biological functions and the activity of enzymes in the body (Nackley et al., 2006). MAP3K5, also known as ASK1, belongs to the MAPK family. ASK1 can be activated by reactive oxygen species to produce a stress response, thereby playing an important role in cell processes such as cell survival and death (Baig et al., 2019). The study by Cho et al. (2012) found that oxidative stress is one of the most effective activators of ASK1 (Cho et al., 2012). In this process, the MKK3/6-p38 MAPK signal performed its biological function, and finally induces the differentiation of cells. The exogenous increase of TNF-α can promote the ASK1-6-P38 MAPK signaling pathway and ultimately regulate the growth of the body. Furthermore, ASK1 activates both JNK and p38 MAPK pathways in response to an array of stresses such as oxidative stress, endoplasmic reticulum stress, and calcium influx (Choi et al., 2011). Therefore, this cell signaling pathway is involved in the process of cell growth and differentiation in animals, thereby promoting the growth and development of animals. As a result, this gene is rarely studied in sheep, but some studies have shown that it is related to cell metabolism and cell differentiation. Therefore, it is necessary to study MAP3K5 g.205261 A > G mutation and body size traits of Hu sheep.
According to the results of qRT-PCR, the MAP3K5 gene was widely expressed in these tissues. The expression level of the heart was significantly higher than those of other tissues, and there was relatively low expression in the rumen. The heart is the central organ of vertebrates, with functions such as contraction, relaxation, and pumping of blood. The role of the heart is to promote blood flow, provide sufficient blood flow to organs and tissues to supply oxygen and various nutrients, and to take away the end products of metabolism so that cells maintain normal metabolism and function. The results of RNA expression profiles were similar to the study of Pu et al. (2016) on Duroc pigs (Pu et al., 2016). At the same time, the research on mouse and chicken embryos of ASK1 by Ferrer-Vaquer et al. (2017) also found similar results (Ferrer-Vaquer et al., 2007). Verhoeff and Mitchell (2017) found that the research on the hearts and lungs was usually combined, so it can be considered that their biological functions are also related and complementary to a certain extent (Verhoeff and Mitchell, 2017). Choi et al. (2011) found the activated ASK1 stimulated MKK3/6–p38MAPK signaling cascade to induce specific myogenic. p38 MAPK is a core signaling molecule in myogenic differentiation (Choi et al., 2011). Chun et al. (2000) demonstrated that p38 MAPK is activated in cardiac myoblast and positively regulates myogenic genes (Chun et al., 2000). Zhang et al. (2016) found that the MLY4 gene has the highest expression level in the hearts of small-tailed Han sheep and Dorper sheep (Zhang et al., 2016). Our results are consistent with previous research. As vital metabolic and immune organs of the body, the heart, liver, and lymph play a vital role in the healthy growth of animals. The liver is a crucial hub for many physiological processes. It is a unique metabolic and immune organ in the body that fully plays the role of oxidation, storage of liver sugar, secretory protein synthesis, trace element metabolism, detoxification, hematopoietic function recovery, and other functions (Racanelli and Rehermann, 2006; Jackson, 2017; Trefts et al., 2017). The lymph circulates through the lymphatic vessels and finally into the veins, through which part of the tissue fluid flows into the blood. Lymph is found in various parts of the animal body and plays a vital role in the body's immune system (Swartz, 2001). Therefore, we speculated that the high expression of liver and lymph can help the heart function better, so that the Hu sheep can maintain a healthy physiological state. The body's metabolism can always be at a normal level, to avoid the influence of diseases, external factors, and other factors that cause Hu sheep to be sub-healthy. Or when confronted with stimulation, the body can metabolize the toxins and other undesirable substances produced by stimulus more quickly, so that Hu sheep can maintain a better mental state (Lu et al., 2019; Orman et al., 2011; Clement et al., 2011). Therefore, the growth of AA-genotype Hu sheep is relatively better than that of GG-genotype Hu sheep. So the sheep's high expression level of the heart, liver, and lymph may be related to high metabolic capacity, highly effective immunity, high-efficiency blood circulation, and metabolism, to enable efficient utilization and processing of nutrients and metabolic wastes in the body, thereby promoting the growth of the animal's body. We speculate that the sheep MAP3K5 gene silent mutation may influence body size traits by participating in the metabolic pathway. The MAP3K5 gene may be used as a candidate gene that affects the body size traits of sheep. And its deep molecular mechanism of action needs further experiments to study and verify it.
In summary, the MAP3K5 g.205261 A > G SNP site was identified in this study, which was related to the body height, body length, chest circumference, and cannon circumference of Hu sheep. The qRT-PCR results indicated that the expression level in the heart was the highest. Therefore, the MAP3K5 gene may be used as a molecular genetic marker to improve the production performance of Hu sheep.
The data are available from the corresponding author upon request.
The supplement related to this article is available online at: https://doi.org/10.5194/aab-66-71-2023-supplement.
XiaZ, WW, and XY conceived and designed the study. LZ, JW, DX, JC, WL, BZ, and CL participated in RNA and DNA extraction. XY, XiwZ, RZ, ZM, JL, and PC analyzed the data. DZ, XL, YukZ, and YuaZ contributed to the feeding experiment and sample collection. XiaZ and XY revised the paper. XY wrote the paper.
The contact author has declared that none of the authors has any competing interests.
In this study, the animal care and experimental procedures were performed in accordance with the rules and guidelines of the government of Gansu People's Congress. The protocols were approved by the Institutional Animal Care and Ethics Committee of Gansu Agricultural University (permit no. 2012-2-159 to conduct animal experiments).
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was supported by the National Joint Research on improved breeds of Livestock and Poultry (grant no. 19210365), the Key Technologies Research and Development Program of Gan Su (grant no. 20YF3NA012), National Natural Science Foundation of China (grant no. 31960653), and Discipline Team Project of Gansu Agricultural University (grant no. GAU-XKTD-2022-20).
This paper was edited by Henry Reyer and reviewed by three anonymous referees.
Abraham, H., Gizaw, S., and Urge, M.: Identification of breeding objectives for Begait goat in western Tigray, North Ethiopia, Trop. Anim. Health Pro., 50, 1887–1892, https://doi.org/10.1007/s11250-018-1640-5, 2018.
Awawdeh, M. S.: Alternative feedstuffs and their effects on performance of Awassi sheep: a review, Trop. Anim. Health Pro., 43, 1297–1309, https://doi.org/10.1007/s11250-011-9851-z, 2011.
Baig, M. H., Baker, A., Ashraf, G. M., and Dong, J. J.: ASK1 and its role in cardiovascular and other disorders: available treatments and future prospects, Expert Rev. Proteomics, 16, 857–870, https://doi.org/10.1080/14789450.2019.1676735, 2019.
Beuzen, N. D., Stear, M. J., and Chang, K. C.: Molecular markers and their use in animal breeding, Vet. J., 160, 42–52, https://doi.org/10.1053/tvjl.2000.0468, 2000.
Chen, T., Wang, L., Li, Q., Long, Y., Lin, Y., Yin, J., Zeng, Y., Huang, L., Yao, T., Abbasi, M. N., Yang, H., Wang, Q., Tang, C., Khan, T. A., Liu, Q., Yin, J., Tu, Q., and Yin, Y.: Functional probiotics of lactic acid bacteria from Hu sheep milk, BMC Microbiol., 20, 228, https://doi.org/10.1186/s12866-020-01920-6, 2020.
Cho, J. H., Lee, M. K., Yoon, K. W., Lee, J., Cho, S. G., and Choi, E. J.: Arginine methylation-dependent regulation of ASK1 signaling by PRMT1, Cell Death Differ., 19, 859–870, https://doi.org/10.1038/cdd.2011.168, 2012.
Choi, T. G., Lee, J., Ha, J., and Kim, S. S.: Apoptosis signal-regulating kinase 1 is an intracellular inducer of p38 MAPK-mediated myogenic signalling in cardiac myoblasts, Biochim. Biophys. Acta, 1813, 1412–1421, https://doi.org/10.1016/j.bbamcr.2011.04.001, 2011.
Chun, Y. K., Kim, J., Kwon, S., Choi, S. H., Hong, F., Moon, K., Kim, J. M., Choi, S. L., Kim, B. S., Ha, J., and Kim, S. S.: Phosphatidylinositol 3-kinase stimulates muscle differentiation by activating p38 mitogen-activated protein kinase, Biochem. Bioph. Res. Co., 276, 502–507, https://doi.org/10.1006/bbrc.2000.3486, 2000.
Clement, C. C., Rotzschke, O., and Santambrogio, L.: The lymph as a pool of self-antigens, Trends Immunol., 32, 6–11, https://doi.org/10.1016/j.it.2010.10.004, 2011.
Clifford, R. J., Edmonson, M. N., Nguyen, C. U., Scherpbier, T., Ying, H. U., and Buetow, K. H.: Bioinformatics Tools for Single Nucleotide Polymorphism Discovery and Analysis, Ann. NY Acad., 1020, 101–109, 2010.
Ferrer-Vaquer, A., Maurey, P., Firnberg, N., Leibbrandt, A., and Neubuser, A.: Expression of ASK1 during chick and early mouse development, Gene Expr. Patterns, 7, 808–816, https://doi.org/10.1016/j.modgep.2007.05.001, 2007.
H, E. E., Ma, L., Xie, X., Ma, J., Ma, X., Yue, C., Ma, Q., Liang, X., Ding, W., and Li, Y.: Genetic polymorphism association analysis of SNPs on the species conservation genes of Tan sheep and Hu sheep, Trop. Anim. Health Pro., 52, 915–926, https://doi.org/10.1007/s11250-019-02063-1, 2020.
Harada, C., Nakamura, K., Namekata, K., Okumura, A., Mitamura, Y., Iizuka, Y., Kashiwagi, K., Yoshida, K., Ohno, S., Matsuzawa, A., Tanaka, K., Ichijo, H., and Harada, T.: Role of apoptosis signal-regulating kinase 1 in stress-induced neural cell apoptosis in vivo, Am. J. Pathol., 168, 261–269, https://doi.org/10.2353/ajpath.2006.050765, 2006.
Hattori, K., Naguro, I., Runchel, C., and Ichijo, H.: The roles of ASK family proteins in stress responses and diseases, Cell Commun. Signal., 7, 9, https://doi.org/10.1186/1478-811X-7-9, 2009.
He, C., Holme, J., and Anthony, J.: SNP genotyping: the KASP assay, Methods Mol. Biol., 1145, 75–86, https://doi.org/10.1007/978-1-4939-0446-4_7, 2014.
Jackson, A. A.: Nutrition and Liver Health, Digest. Dis., 35, 411–417, https://doi.org/10.1159/000456596, 2017.
Kyriakis, J. M. and Avruch, J.: Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update, Physiol. Rev., 92, 689–737, https://doi.org/10.1152/physrev.00028.2011, 2012.
Li, M., Xia, H., Chen, D., Ji, D., Kenji, T., Li, R., Liao, X., Mao, Y., Sun, W., Geng, R., and Yang, Z.: Genetic differentiation and phylogeny of 27 sheep populations based on structural gene loci, Mol. Cell. Probe., 37, 55–59, https://doi.org/10.1016/j.mcp.2017.11.006, 2018.
Livak, K. J. and Schmittgen, T. D.: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods, 25, 402–408, https://doi.org/10.1006/meth.2001.1262, 2001.
Lu, Z., Xu, Z., Shen, Z., Shen, H., and Aschenbach, J. R.: Transcriptomic analyses suggest a dominant role of insulin in the coordinated control of energy metabolism and ureagenesis in goat liver, BMC Genomics, 20, 854, https://doi.org/10.1186/s12864-019-6233-9, 2019.
Nackley, A., Shabalina, S., Tchivileva, I., Satterfield, K., Korchynskyi, O., Makarov, S., Maixner, W., and Diatchenko, L.: Human Catechol-O-Methyltransferase Haplotypes Modulate Protein Expression by Altering mRNA Secondary Structure, Science, 314, 1930–1933, 2006.
Orman, M. A., Berthiaume, F., Androulakis, I. P., and Ierapetritou, M. G.: Pathway analysis of liver metabolism under stressed condition, J. Theor. Biol., 272, 131–140, https://doi.org/10.1016/j.jtbi.2010.11.042, 2011.
Pedersen, L. D., Sorensen, A. C., and Berg, P.: Marker-assisted selection can reduce true as well as pedigree-estimated inbreeding, J. Dairy Sci., 92, 2214–2223, https://doi.org/10.3168/jds.2008-1616, 2009.
Pu, L., Zhang, L. C., Zhang, J. S., Song, X., Wang, L. G., Liang, J., Zhang, Y. B., Liu, X., Yan, H., Zhang, T., Yue, J. W., Li, N., Wu, Q. Q., and Wang, L. X.: Porcine MAP3K5 analysis: molecular cloning, characterization, tissue expression pattern, and copy number variations associated with residual feed intake, Genet. Mol. Res., 15, gmr.15037998, https://doi.org/10.4238/gmr.15037998, 2016.
Quan, K., Li, J., Han, H., Wei, H., Zhao, J., Si, H. A., Zhang, X., and Zhang, D.: Review of Huang-huai sheep, a new multiparous mutton sheep breed first identified in China, Trop. Anim. Health Pro., 53, 35, https://doi.org/10.1007/s11250-020-02453-w, 2020.
Racanelli, V. and Rehermann, B.: The liver as an immunological organ, Hepatology (Baltimore, Md.), 43, 54–62, https://doi.org/10.1002/hep.21060, 2006.
Sirotkin, A. V., Benco, A., Tandlmajerova, A., Vasicek, D., Kotwica, J., Darlak, K., and Valenzuela, F.: Transcription factor p53 can regulate proliferation, apoptosis and secretory activity of luteinizing porcine ovarian granulosa cell cultured with and without ghrelin and FSH, Reproduction, 136, 611–618, https://doi.org/10.1530/REP-08-0229, 2008.
Swartz, M. A.: The physiology of the lymphatic system, Adv. Drug Deliver. Rev., 50, 3–20, https://doi.org/10.1016/s0169-409x(01)00150-8, 2001.
Takenaka, S., Fujisawa, T., and Ichijo, H.: Apoptosis signal-regulating kinase 1 (ASK1) as a therapeutic target for neurological diseases, Expert Opin. Ther. Tar., 24, 1061–1064, https://doi.org/10.1080/14728222.2020.1821648, 2020.
Trefts, E., Gannon, M., and Wasserman, D. H.: The liver, Curr. Biol., 27, R1147–R1151, https://doi.org/10.1016/j.cub.2017.09.019, 2017.
Verhoeff, K. and Mitchell, J. R.: Cardiopulmonary physiology: why the heart and lungs are inextricably linked, Adv. Physiol. Educ., 41, 348–353, https://doi.org/10.1152/advan.00190.2016, 2017.
Wang, W., Cheng, L., Guo, J., Ma, Y., and Li, F.: Expression of Ghrelin in gastrointestinal tract and the effect of early weaning on Ghrelin expression in lambs, Mol. Biol. Rep., 41, 909–914, https://doi.org/10.1007/s11033-013-2935-2, 2014.
Zhang, C., Wang, J., Wang, G., Ji, Z., Hou, L., Liu, Z., and Chao, T.: Molecular cloning and mRNA expression analysis of sheep MYL3 and MYL4 genes, Gene, 577, 209–214, https://doi.org/10.1016/j.gene.2015.11.041, 2016.
Zhao, H., Wu, X., Cai, H., Pan, C., Lei, C., Chen, H., and Lan, X.: Genetic variants and effects on milk traits of the caprine paired-like homeodomain transcription factor 2 (PITX2) gene in dairy goats, Gene, 532, 203–210, https://doi.org/10.1016/j.gene.2013.09.062, 2013.