the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The effect of propolis addition to the laying-hen diet on performance, serum lipid profile and liver fat rate
Şaziye Canan Bölükbaşı
Hilal Ürüşan
Betül Apaydın Yıldırım
The aim of this study was to evaluate the effect of propolis (P) on performance, egg quality parameters, serum lipid profile, some liver enzymes and liver fat ratio. One-hundred-and-twenty Lohmann (LSL) laying hens were divided into five groups, and each group consisted of six subgroups. The control group was fed basal diet. The other groups were fed high-energy (HE) diets to induce fatty liver syndrome, and 0, 100, 200 and 300 mg kg−1 of propolis were supplemented with high-energy feeds. During the 8-week trial, feed and water were given ad libitum.
It was determined that egg production and feed conversion ratio were decreased in the high-energy feed group without the addition of propolis. The highest egg production was found in HE + 100 and HE + 200 mg kg−1 of P groups. It was found that liver fat ratios were higher in the group fed with HE + 0 mg kg−1 of P feed (P<0.01) than other groups. But the addition of P decreased the liver fat rate significantly. The highest very low density lipoprotein (VLDL), triglyceride (TG) and low-density lipoprotein (LDL) values were found for the HE + 0 mg kg−1 of P group. The addition of 200 mg kg−1 of P to high-energy feed increased glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) values.
In conclusion, high-energy feed adversely affected egg production and liver fat ratio, but the addition of 100 or 200 mg kg−1 of propolis improved egg production and decreased liver fat ratio.
- Article
(416 KB) - Full-text XML
- BibTeX
- EndNote
In order to meet egg requirements, which have an important place in human nutrition, the caged layer hen system is widely practiced today. In caged layer hens, the amount of product obtained is high because more animals are housed per unit area. However, this situation causes some problems such as fatty liver syndrome. For laying hens raised in cages, fat can occur in the liver due to the restriction in the movement area and the high-energy value of the feeds (Shini et al., 2019). “Fatty liver”, which is called hepatosteatosis in medical language, means excessive accumulation of fat in liver cells. Fatty liver is a disease characterized by an accumulation of fat in the abdominal cavity and a decrease in egg production. The disease causes a shrinkage of egg numbers in laying hens, a drop of 45 % in egg production within a few days and subsequent deaths (Crespo et al., 2013). It was reported that the amount of liver fat is significantly affected by dietary fat (Butler, 1976).
This disease, which is associated with feeding birds with a high-energy feed, is most common during the summer period. The liver is usually enlarged, pale and fragile. Although there is no known method for the treatment of fatty liver syndrome, its development can be prevented by the use of lipotropic agents such as vitamin E, vitamin B12 and choline chloride.
Propolis (P) is a resinous substance collected by bees from leaves, stems and buds of various plants, mixed with bee enzymes, pollen and wax. Propolis is composed of many components, primarily polyphenol components, such as phenolic acids, esters, phenolic aldehydes and ketones. The antiseptic, antibacterial, anti-inflammatory, immunomodulatory, antioxidant, antimutagenic and cytotoxic effects of propolis have been proven in scientific studies. In addition, its protective effect on liver has been reported by many researchers (Bhadauria et al., 2009; Kısmet et al., 2008; Paulino et al., 2014; Wali et al., 2015; Omar et al., 2016). Most of the biological effects of propolis are related to its antioxidant capacity.
To the best of our knowledge, there is no study on the effect of propolis on fatty liver in laying hens. In this study, in order to see the effect of propolis more clearly, we aimed to examine the effect of propolis supplementation on performance, liver fat ratio, egg quality and some antioxidant enzymes by promoting fatty liver in layer hens fed high-energy feed.
2.1 Experimental animals and experimental design
The study was conducted in accordance with the ethics committee principles of Atatürk University veterinary faculty (2018/1). The aim of this study was to evaluate the effect of propolis on performance and fatty liver in laying hens. Laying hens were fed with a high-energy feed (2850 kcal kg−1) to induce fatty liver. One-hundred-and-twenty white Lohmann (LSL) laying hens at an age of 70 weeks were used. The hens were divided into five groups, and each group consisted of six subgroups. The birds were placed in four-story battery cages ( cm) with six hens in each as replicates. The first group was the control group where the birds were fed with basal feed (Table 1), while the other groups were fed with feeds that added 0, 100, 200 and 300 mg kg−1 of propolis (P) to high-energy feeds. During the 8-week trial, feed and water were given ad libitum. The propolis used in the diet was obtained from a commercial company.
Table 1Composition of feeds used in the trial (%).
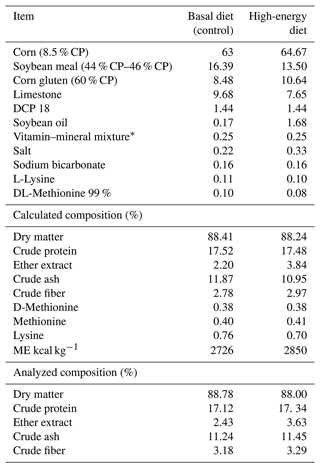
CP: crude protein. ME: metabolic energy. DCP: dicalcium phosphate. IU: international unit. * per kilogram diet added: 12 000 IU vitamin A; 2500 IU vitamin D3; 30 IU vitamin E; 4 mg vitamin K3; 3 mg vitamin B1; 6 mg vitamin B2; 30 mg niacin; 10 mg calcium D-pantothenate; 5 mg vitamin B6; 0.015 mg vitamin B12; 1 mg folic acid; 0.050 mg D-biotin; 50 mg vitamin C; 300 mg choline chloride; 80 mg manganese; 60 mg iron; 60 mg zinc; 5 mg copper; 0.5 mg cobalt; 0.2 mg iodine; 0.15 mg selenium.
2.2 Performance analysis
Feed consumption, egg production, egg weight and feed conversion ratio (kg feed kg−1 egg) values of the birds were determined with the measurements made every 2 weeks. Likewise, egg quality criteria such as shell thickness, breaking strength, albumen ratio, yolk ratio, shell ratio, shape index and Haugh unit were determined by the measurements made every 2 weeks.
2.3 Blood parameter analysis
At the end of the experiment, blood samples that were taken from the vein of one animal from each subgroup (n=6) and placed into heparinized tubes were centrifuged (3000 rpm for 10 min), and the samples were stored at −80 ∘C.
Superoxide dismutase (SOD) activity (Sun et al., 1988), glutathione (GSH) level (Tietze, 1969), MDA (malondialdehyde) level (Yoshioka et al., 1979), glutathione peroxidase (GPx) activity (Matkovics et al., 1988), catalase (CAT) activity (Goth, 1991), TP (total protein) levels (Lowry, 1951) and non-esterified fatty acid (NEFA) levels in plasma (Biont Chicken NEFA ELISA Kit, cat. no. YLA0179CH) were measured with a BioTek ELISA reader (BioTek μQuant MQX200 ELISA reader, USA). TP levels were used to calculate the SOD and GPx activity. Plasma cholesterol, glucose, LDL (low-density lipoprotein), HDL (high-density lipoprotein), VLDL (very low density lipoprotein), AST (aspartate transaminase), ALT (alanine transaminase) and TG (triglyceride) values were analyzed in a special laboratory.
At the end of the experiment, one animal from each subgroup was slaughtered. Liver wet weights were determined (n=6); then they were brought to the laboratory and dried at 105 ∘C where their dry weights were determined. Then, the fat ratio was determined by ether extraction in the dried samples (Kutlu, 2008).
2.4 Statistical analyses
For performance values, egg quality criteria, some blood parameters and antioxidant enzyme values, variance analyses were performed by the “general linear model” procedure, and the importance controls of the important data were performed using the SPSS 17 program. Differences between groups were found by Duncan's multiple comparison test.
The values of the feed consumption, egg weight, egg production and feed conversion ratio of the experimental groups are given in Table 2.
No significant difference was found between the control group, the HE + 0 mg kg−1 of P group and the HE + 300 mg kg−1 of P group in terms of feed intake. However, it was determined that adding 100 and 200 mg kg−1 of P to feed increased feed intake significantly (P<0.05). There was no significant difference between groups in terms of egg weight. It was determined that egg production was significantly lower in HE + 0 and HE + 300 mg kg−1 of P groups compared to the control group, but the addition of 100 and 200 mg kg−1 of P to a high-energy diet significantly increased egg production (P<0.01). In the HE + 0 mg kg−1 of P and HE + 300 mg kg−1 of P groups, the degree of feed conversion ratio decreased significantly (P<0.05).
Table 2The effect of propolis on the performance of laying hens consuming high-energy feed.
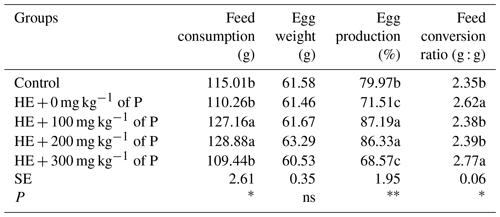
a–c: the averages shown with different letters in the same column are different from each other. HE: high energy. P: propolis. Control: basal-fed group. HE + 0 mg kg−1 of P: high-energy-fed group + 0 mg kg−1 of propolis. HE + 100 mg kg−1 of P: high-energy-fed group + 100 mg kg−1 of propolis. HE + 200 mg kg−1 of P: high-energy-fed group + 200 mg kg−1 of propolis. HE + 300 mg kg−1 of P: high-energy-fed group + 300 mg kg−1 of propolis. SE: standard error. ns: not significant. * P<0.05. P<0.01.
There was no significant difference between the groups in terms of values of egg quality criteria such as albumen ratio, yolk ratio, shell ratio, Haugh unit, shell breaking strength or shell thickness (Table 3).
Table 3The effect of propolis on egg quality of laying hens consuming high-energy feed.
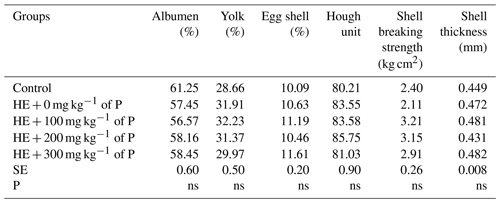
a–c: the averages shown with different letters in the same column are different from each other. HE: high energy. P: propolis. Control: basal-fed group. HE + 0 mg kg−1 of P: high-energy-fed group + 0 mg kg−1 of propolis. HE + 100 mg kg−1 of P: high-energy-fed group + 100 mg kg−1 of propolis. HE + 200 mg kg−1 of P: high-energy-fed group + 200 mg kg−1 of propolis. HE + 300 mg kg−1 of P: high-energy-fed group + 300 mg kg−1 of propolis. SE: standard error. ns: not significant.
The means of wet weight, dry weight and fat ratio of liver are given in Table 4. The lowest values in terms of wet and dry liver weights were determined in the control and HE + 300 mg kg−1 of P groups. The difference among the experimental groups in terms of liver fat ratio was significant (P<0.01); the highest value was found in the HE + 0 mg kg−1 of P group, while the lowest value was observed in the HE + 300 mg kg−1 of P group. Liver fat ratios of the HE + 100 and HE + 200 mg kg−1 of P groups were found to be considerably lower than the HE + 0 mg kg−1 of P group.
Table 4The effect of propolis on wet weight (g), dry weight (g) and fat ratio (%) of liver of laying hens consuming high-energy feed.
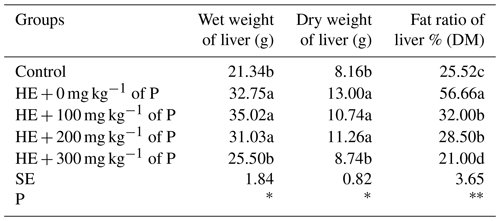
a–c: the averages shown with different letters in the same column are different from each other. DM: dry matter. HE: high energy. P: propolis. Control: basal-fed group. HE + 0 mg kg−1 of P: high-energy-fed group + 0 mg kg−1 of propolis. HE + 100 mg kg−1 of P: high-energy-fed group + 100 mg kg−1 of propolis. HE + 200 mg kg−1 of P: high-energy-fed group + 200 mg kg−1 of propolis. HE + 300 mg kg−1 of P: high-energy-fed group + 300 mg kg−1 of propolis. SE: standard error. ns: not significant. * P<0.05. P<0.01.
There was no significant difference between the groups in terms of ALT, AST, glucose, total cholesterol and HDL cholesterol. The difference between the groups in terms of VLDL, TG and LDL cholesterol was significant (P<0.01), with the highest values seen in the HE + 0 mg kg−1 of P group. It was observed that the NEFA level increased significantly in the HE + 300 mg kg−1 of P group.
Table 5The effect of propolis on some blood plasma biochemistry parameters of laying hens consuming high-energy feed.
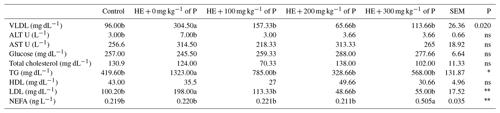
a–c: the averages shown with different letters in the same row in the are different from each other. HE: high energy. P: propolis. Control: basal-fed group. HE + 0 mg kg−1 of P: high-energy-fed group + 0 mg kg−1 of propolis. HE + 100 mg kg−1 of P: high-energy-fed group + 100 mg kg−1 of propolis. HE + 200 mg kg−1 of P: high-energy-fed group + 200 mg kg−1 of propolis. HE + 300 mg kg−1 of P: high-energy-fed group + 300 mg kg−1 of propolis. SE: standard error. ns: not significant. * P<0.05. P<0.01.
There was a significant difference (P<0.01) between the groups in terms of MDA, GSH, SOD, CAT and GPx values (Table 6). The highest plasma MDA value and the lowest GSH, SOD, CAT and GPx values were detected in the HE + 300 mg kg−1 of P group.
Contrary to expectations, the MDA value in this study was determined to be the highest in the HE + 300 mg kg−1 of P group, not in the HE + 0 % P group. It was observed that MDA levels increased significantly in parallel with the NEFA value in the HE + 300 mg kg−1 of P group (Table 5).
Table 6The effect of propolis on MDA and some enzyme activity of liver of laying hens consuming high-energy feed.
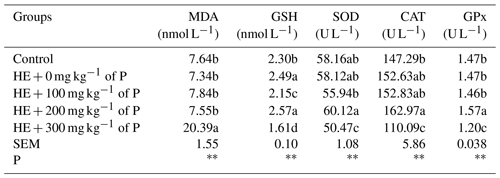
a–d: the averages shown with different letters in the same column are different from each other. HE: high energy. P: propolis. Control: basal-fed group. HE + 0 mg kg−1 of P: high-energy-fed group + 0 mg kg−1 of propolis. HE + 100 mg kg−1 of P: high-energy-fed group + 100 mg kg−1 of propolis. HE + 200 mg kg−1 of P: high-energy-fed group + 200 mg kg−1 of propolis. HE + 300 mg kg−1 of P: high-energy-fed group + 300 mg kg−1 of propolis. SE: standard error. ns: not significant. P<0.01.
It has been reported that propolis has antioxidant, antimutagenic and immunomodulatory properties, and these properties are due to its rich flavonoid, phenolic acid and terpenoid content (Prytzyk et al., 2003; Wang et al., 2003). The antioxidant properties of propolis are thought to be the cause of the increase in egg production and feed conversion ratio. In addition, the increase in feed consumption has been attributed to the aroma and anti-lipidemic effect of propolis. Similarly to this study, Galal et al. (2008) reported that the addition of propolis to laying-hen rations increased feed consumption, egg production, egg weight and improved feed efficiency. El-Neney and Awadien (2016) reported that layer hens fed diets supplemented with different levels of propolis (0.1, 0.2 or 0.3 g kg−1) significantly improved feed efficiency per hen, egg production, egg weight and egg mass. It has been reported that the addition of 1 g kg−1 of P in Japanese quails (Denli et al., 2005) and 2 g kg−1 of P in ducks improved feed conversion ratio (Abdel-Rahman and Mosaad, 2013). Abdel-Kareem and El-Sheikh (2017) found that adding different levels of propolis to laying-hen feed did not affect feed intake but increased egg production and feed conversion ratio. Contrary to these studies, Belloni et al. (2015) reported that as the level of dietary propolis content (1.0 % to 3.0 %) in the layer's diet increased, feed intake decreased. They suggested that this decrease in feed consumption may be due to the aroma of propolis. Navarro-Villa et al. (2019) reported that egg production decreased significantly in laying hens fed with high-energy and low-protein feed to promote liver formation.
In this study, no effect of propolis on egg quality criteria was detected. Similarly, it has been reported that the addition of propolis to laying-hen rations does not affect the albumen, yolk or egg shell ratio but does increase the Hough unit and shell thickness (Galal et al., 2008). Özkök et al. (2013) reported that propolis doses (0.1, 0.2 or 0.4 g kg−1) had no dietary effect on quality criteria such as Haugh units or shell thickness. However, Vilela et al. (2012) reported that propolis has a positive effect on the internal content of egg and quality of the egg shell. Abdel-Kareem and El-Sheikh (2017) reported that the addition of 1000 mg kg−1 of P to laying-hen diets increased the weight of yolk and shell and the Haugh unit but did not affect albumen weight.
It was determined that the addition of propolis to the diet increased the wet and dry weights of the liver in this study. Similarly, Hassan and Abdulla (2011) found that the addition of 400 mg kg−1 of P to broiler diets increased liver weight.
It has been reported that the rate of liver fat exceeds 40 % of the dry weight and can even reach 70 % in the case of fatty liver (Ivy and Nesheim, 1973). In the present study, it was determined that the liver fat ratio (56.66 %) was higher in the group fed with high-energy feed (HE + 0 % P) compared to the other groups. Zhuang et al. (2019) observed that the rate of liver fat increased significantly in their study in which they fed laying hens with a high-energy low-protein diet to induce fatty liver hemorrhagic syndrome. Similarly, in many studies conducted in previous years, it was reported that the rate of liver fat increased in animals fed with high-energy feed (Splittgerber et al., 1969; Jensen et al., 1970; Akkılıç and Tanyolaç, 1975). Rozenboim et al. (2016) reported that in laying hens fed a high-fat diet, the liver fat rate in young animals was not affected by diet, but the liver fat rate in old animals was lower than in the control group. Unlike mammals, fat synthesis is high in the liver of birds (Hermier, 1997). The liver plays a major role in fat synthesis and metabolism in laying hens. Similar to mammals, in avian species the digestion and absorption of dietary fat occurs in the small intestine (Tancharoenrat et al., 2014). However, due to the poorly developed intestinal lymphatic system in birds, dietary fatty acids are discharged directly into the portal blood system (instead of the lymphatic system) in the form of very low density lipoproteins (VLDL) called “portomicrons” (Bensadoun and Rothfeld, 1972). Bird livers become more prone to fat accumulation, as most portomicrons travel from the portal blood system to the liver before reaching the rest of the circulation (Cherian et al., 2002).
Similar to this study, Navarro-Villa et al. (2019) reported that liver fat ratio increased significantly in laying hens fed with high-energy and low-protein feed to promote fatty liver formation. In the current study, it was determined that adding propolis to the high-energy diet significantly reduced the liver fat ratio compared to the control group. Lin et al. (1997) reported that 30 mg kg−1 of P can prevent fatty liver degeneration caused by prolonged alcohol intake in humans.
Studies on the properties of propolis have shown that its addition to the diet protects liver tissue against the negative effects of various hepatotoxic factors (Banskota et al., 2000; Bazo et al., 2002; Bhadauria et al., 2009).
Unlike this study, in a study in which different levels of propolis were added to laying-hen diets, it was reported that a level of 1000 mg kg−1 of P increased total protein level and decreased ALT, AST and cholesterol levels (Abdel-Kareem and El-Sheikh, 2017). Galal et al. (2008) reported that blood triglyceride, cholesterol and ALT levels were significantly reduced in laying hens consuming 100–150 mg kg−1 of P. Zhuang et al. (2019) found that the amount of ALT increased significantly in layer hens fed with a high-energy low-protein diet compared to the control group. In a study by Attia et al. (2014), it was reported that blood triglyceride and cholesterol concentrations were significantly reduced in chickens supplemented with 300 mg kg−1 of P continuously for 35 d. The researchers argued that the decrease in these values was beneficial and safe by minimizing the liver function and reducing the harmful effects on the tissues by propolis treatment.
Koya-Miyata et al. (2009) found that propolis (5 mg kg−1 for 10 d) significantly reduced triglyceride, cholesterol, and NEFA levels in high-fat-diet mice. Hashem et al. (2013) reported in a study they conducted on rabbits that the addition of 150 mg kg−1 of P reduced cholesterol and triglyceride levels; it had no effect on LDL cholesterol and increased HDL cholesterol. In a study conducted in patients with non-alcoholic fatty liver disease, it was reported that the addition of propolis to the diet reduced the level of total cholesterol (Nikbaf-Shandiz et al., 2022). Kısmet et al. (2017) reported in their study on mice with non-alcoholic fatty liver syndrome that the addition of 100 and 200 mg of propolis to the ration reduced total cholesterol, triglyceride, HDL cholesterol, ALT and AST levels in serum.
In fatty liver symptom, overload of the non-esterified fatty acid (NEFA) level has been reported to trigger reactive oxidative stress formation through mitochondria-dependent oxidation or microsomal enzymes (Kısmet et al. 2017). Contrary to this study, Hashem et al. (2013) reported that the addition of 150 mg kg−1 of P reduced MDA level in rabbits.
Catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) are involved in the enzymatic antioxidant defense system of the body (Arya et al., 2021). While the highest SOD, CAT and GPx values were found in the 200 mg kg−1 of P group, the lowest values were found in the 300 mg kg−1 of P group in this study. And the lowest GSH value was determined in the 300 mg kg−1 of P group.
On the other hand, Arya et al. (2021) reported in a study they conducted on patients with non-alcoholic fatty liver syndrome that SOD and GPx levels were lower than the control group, and the MDA value was higher. Similar to this study, it has been reported that there is a negative correlation between MDA and SOD in some other studies (Koruk et al., 2004; Videla et al., 2004).
As a result, it was determined that the liver fat ratio increased significantly and egg production decreased in laying hens fed with a high-energy feed. However, it has been determined that the addition of 200 mg kg−1 of propolis to the high-energy diet increases egg production; significantly reduces the liver fat rate; decreases the amounts of LDL and triglycerides; and increases SOD, CAT, and GPx activities. It has been determined that especially 200 mg kg−1 of propolis may be used in laying-hen rations since it has a positive effect on many parameters, but it has also been concluded that more studies are needed on the subject.
The data presented in this study are available free of charge for any user upon reasonable request from the corresponding author.
ŞCB and HÜ designed the study, ŞCB performed the statistical analysis, ŞCB, HÜ and BAY made laboratory analyzes and ŞCB wrote the paper.
The contact author has declared that none of the authors has any competing interests.
The study was conducted in accordance with the ethics committee principles of Atatürk University veterinary faculty (no. 2018/1).
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was edited by Manfred Mielenz and reviewed by Unal Kilic and one anonymous referee.
Abdel-Kareem, A. A. A. and El-Sheikh, T. M.: Impact of supplementing diets with propolis on productive performance, egg quality traits and some haematological variables of laying hens, J. Anim. Physiol. Anim. Nutr., 101, 441–448, 2017.
Abdel-Rahman, M. A. and Mosaad, G. M.: Effect of propolis as additive on some behavioural patterns, performance and blood parameters in Muscovy broiler ducks, J. Adv. Vet. Res., 3, 64–68, 2013.
Akkılıç, M. and Tanyolaç, A.: Kafeste beslenen tavuk rasyonlarındaki enerji düzeyinin karaciğer yağlanması üzerine etkisi, J. Ankara Univ. Vet. Fac., 71, 370–389, 1975.
Arya, A., Azarmehr, N., Mansourian M., and Doustimotlagh, A. M.: Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: a combined molecular docking approach to clinical studies, Arch. Physiol. Biochem., 127, 557–564, https://doi.org/10.1080/13813455.2019.1659827, 2021.
Attia, Y. A., Al-Hamid, A. E. A., Ibrahim, M. S., Al-Harthi, M. A., Bovera, F., and Elnaggar, A. S.: Productive performance, biochemical and hematological traits of broiler chickens supplemented with propolis, bee pollen, and mannan oligosaccharides continuously or intermittently, Livestock Sci., 164, 87–95, 2014.
Banskota, A. H., Tezuka, Y., Adnyana, I. K., Midorikawa, K., Matsushige, K., Message, D., Huertas, A. A. G., and Kadota, S.: Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China, J. Ethnopharmacol., 72, 239–246, 2000.
Bazo, A. P., Rodriges, M. A. M., Sforcin, J. M., de Camargo, J. L. V., Ribeiro, L. R., and Salvadori, D. M. F.: Protective action of propolis on the rat colon carcinogenesis, Tera Carcin Mutagen, 22, 183–194, 2002.
Belloni, M., Almeida, P. I. C. L., Nääs, I. A., Alves, M. C. F., Garcia, R. G., Caldara, F. R., and Seno, L. O.: Produc-tive, qualitative, and physiological aspects of layer hens fed with propolis, Braz. J. Poultry Sci., 17, 467–472, 2015.
Bensadoun, A. and Rothfeld, A.: The form of absorption of lipids in the chicken, Gallus domesticus, Proc. Soc. Exp. Biol. Med., 141, 814–817, 1972. [CrossRef]
Bhadauria, M. and Nirala, S. K.: Reversal of acetaminophen induced subchronic hepatorenal injury by propolis extract in rats, Environ. Toxicol. Pharmacol., 27, 17–25, 2009.
Butler, E.: Lipid metabolism in the fowl under normal and abnormal circumstances, Proc. Nutr. Soc., 34, 29–34, 1975.
Cherian, G., Holsonbake, T. B., Goeger, M. P., and Bildfell, R.: Dietary CLA alters yolk and tissue FA composition and hepatic histopathology of laying hens, Lipids, 37, 751–757, 2002.
Crespo, R. and Shivaprasad, H. L.: Developmental, metabolic, and other noninfectious disorders, in: Diseases of Poultry, 11th Edn., Iowa State Press, Ames, IA, USA, 1233–1270, edited by: Swayne, D. E., ISBN: 9780470958995, https://doi.org/10.1002/9781119421481.ch30, 2013.
Denli, M., Çankaya, S., Silici, S., Okan, F., and Uluocak, A. N.: Effect of dietary addition of Turkish propolis on the growth performance, carcass characteristics and serum variables of quail (Coturnix coturnix japonica), Asian-Austral. J. Animi. Sci., 18, 848–854, https://doi.org/10.5713/ajas.2005.848, 2005.
El-Neney, B. A. and Awadien, N. B.: The use of propolis as a source of natural additives to improve the productive performance and 40 immune system of chickens local, 2 – Effect on growing Dokki4, 9th International Poultry Conference-Proceedings, organized by Animal Production Research Institute (APRI), Ain Sukhna, Egypt, 62–78, 7–10 November, https://www.researchgate.net/publication/320258260 (last access: 12 March 2023), 2016.
Galal, A., Abd El-Motaal, A. M., Ahmed, A. M. H., and Zaki, T. G.: Productive performance and immune response of laying hens as affected by dietary propolis supplementation, Int. J. Poult. Sci., 7, 271–278, 2008.
Goth, L.: A simple method for determi-nation of serumcatalase activity and revision of reference range, Clin. Chim. Ac., 196, 143–152, 1991.
Hashem, N. M., Abd El-Hady, A., and Hassan, O.: Effect of vitamin E or propolis supplementation on semenquality, oxidative status and hemato-biochemical changesof rabbit bucks during hot season, Livestock Sci., 157, 520–526, 2013.
Hassan, M. and Abdulla, T.: The effect of propolis feed supplementation on hygiene and performance of broiler chickens, Iraqi. J. Vet. Sci., 25, 77–82, 2011.
Hermier, D.: Lipoprotein metabolism and fattening in poultry, J. Nutr., 127, 805–808, 1997.
Ivy, C. A. and Nesheim, M. C.: Factors influencing the liver fat content of laying hens, Poultry Sci., 52, 281–291, 1973.
Jensen, L. S., Sehuınaier, G. W., Funk, A. D., and Smith, T. C.: A new lipotl'llpir agelZtfor tlıe lay'ing Izen, Poultry Sci., 49, 1401, I970.
Kısmet, K., Sabuncuoglu, M. Z., Kilicoglu, S. S., Kilicoglu, B., Devrim, E., Erel, S., Sunay, A. E., Erdemli, E., Durak, I., and Akkus, M. A.: Effect of propolis on oxidative stress and histomorphology of liver tissue in experimental obstructive jaundice, Eur. Surg. Res., 41, 231–237, 2008.
Kısmet, K., Ozcan, Ç., Kuru, S., Celemli, O. G., Celepli, P., Senes, M., Güçlü, T., Sorkun, K., Hucumenoglu, S., and Besler, T.: Does propolis have any effect on non-alcoholic fatty liver disease, Biomed. Pharmacother., 90, 863–871, 2017.
Koruk, M., Taysi, S., Savaş, M. C., Yılmaz, O., Akçay, F., and Karakök, M.: Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis, Ann. Clin. Lab. Sci., 34, 57–62, 2004.
Koya-Miyata, S., Arai, N., Mizote, A., Taniguchi, Y., Ushio, S., Iwaki, K., and Fukuda, S.: Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice, Biol Phar. Bul., 32, 2022–2028, 2009.
Kutlu, H.: Yem Değerlendirme ve Analiz Yöntemleri. Çukurova University, Agricultural Faculty, Animal Science Department, Lecture Notes, Adana-Turkey, 7, 12–76, http://www.zootekni.org.tr/upload/File/sunular/tm.pdf (last access: 16 November 2022), 2008.
Lin, S. C., Lin, Y. H., Chen, C. F., Chung, C. Y., and Hsu, S. H.: The hepatoprotective and therapeutic effects of propolis ethanol extract on chronic alcohol-induced liver injuries, Am. J. Chin. Med., 25, 325–332, https://doi.org/10.1142/S0192415X97000366, 1997.
Lowry, O. H., Rose Brough, N. J., Farr, A. L., and Randall, V. J.: Protein Measurement with the Folin Phenol, J. Biol. Chem., 193, 265–275, https://doi.org/10.1016/S0021-9258(19)52451-6, 1951.
Matkovics, B., Szabo, L., and Varga, I. S.: Determination of enzyme activities in lipid peroxidation and glutathione pathways, Lab. Diagnoszt., 15, 248–249, 1988.
Navarro-Villa, A., Mica, J. H., de los Mozos, J., den Hartog, L. A., and García-Ruiz, A. I.: Nutritional dietary supplements to reduce the incidence of fatty liver syndrome in laying hens and the use of spectrophotometry to predict liver fat content, J. Appl. Poult. Res., 28, 435–446, https://doi.org/10.3382/japr/pfz005, 2019.
Nikbaf-Shandiz, M., Tutunchi, H., Khoshbaten, M., Bonaba, H. N., and Ebrahimi-Mameghan, M.: Propolis supplementation in obese patients with non-alcoholic fatty liver disease: effects on glucose homeostasis, lipid profile, liver function, anthropometric indices and meta-inflammation, Food Funct., 13, 11568–11578, 2022.
Omar, N. A. A., Allithy, A. N. E. A., Baghdadi, H., Zolaly, M., Abdel-haleem, M., Helmy, M. M., Ayat, M. M. A., and El Sayed, S. M.: Hepatoprotective effects exerted by propolis against doxorubicin-induced rat liver toxicity: a biochemical and histopathological study, Am. J. Cancer Prev., 4, 36–40, 2016.
Özkök, D., Iscan, K. M., and Ilici, S.: Effects of di-etary propolis supplementation on performance and egg quality in laying hens, J. Anim. Vet. Adv., 12, 269–275, 2013.
Paulino, N., Barbosa, A. P., Paulino, A. S., and Marcucci, M. C.: Hepatoprotective effect of green propolis is related with antioxidant action in vivo and in vitro, Oxid. Antioxid. Med. Sci., 3, 43–50, 2014.
Prytzyk, E., Dantas, A. P, Salomao, K., Pereira, A. S., Bankova, V. S., Castro, S. L. D., and Neto, F. R. A.: Flavonoids and trypanocidal activity of bulgarian propolis, J. Ethnopharmacol., 88, 189–193, 2003.
Rozenboim, I., Mahato, J., Cohen, N. A., and Tirosh, O.: Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens, Poultry Sci., 95, 612–621, 2016.
Shini, A., Shini, S., and Bryden, W. L.: Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system, Avian Pathol., 48, 25–34, https://doi.org/10.1080/03079457.2018.1538550, 2019.
Splittgerber, H., Wein, F. K., and Arhelger, R.: Untersuebungen über die Hö'he des Fettgehaltes der Lebern von Hemzen bei versehiedenen Futlermisc!wngen, Deutsche Geflügclwirtschaft, 12, 1021–1022, 1969.
Sun, Y., Oberley, L. W., and Li, Y. A.: Simple method for clinical assay of superoxide dismutase, Clin. Chem., 34, 497–500, 1988.
Tancharoenrat, P., Ravindran, V., Zaefarian, F., and Ravindran, G.: Digestion of fat and fatty acids along the gastrointestinal tract of broiler chickens, Poult. Sci., 93, 371–379, 2014.
Tietze, F.: Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione, applications to mammalian blood and other tissues, Anal. Biochem., 27, 502–522, 1969.
Videla, L. A., Rodrigo, R., Orellana, M., Fernandez, V., Tapia, G., Quiñones, L., Varela N., Contreras, J., Lazarte, R., Csendes, A., Rojas, J., Maluenda, F., Burdiles, P., Diaz, J. C., Smok, G., Thielemann, K., and Poniachik, J.: Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients, Clin. Sci., 106, 261–268, 2004.
Vilela, C., Vargas, G., Fischer, G., Ladeira, S., de Faria, R., Nunes, C., Lima, M., Hübner, S., Luz, P., and Osório, L.: Propolis: a natural product as an alternative for disinfection of embryonated eggs for incubation, Arq. Inst. Biol., 79, 161–167, https://doi.org/10.1590/S1808-16572012000200003, 2012.
Wali, A. F., Avula, B., Ali, Z., Khan, I. A., Mushtaq, A., Rehman, M. U., Akbar, S., and Masoodi, M. H.: Antioxidant, hepatoprotective potential and chemical profiling of propolis ethanolic extract from Kashmir Himalaya region using UHPLC-DAD QToF-MS, Biomed. Res. Int., 2015, 393–462, https://doi.org/10.1155/2015/393462, 2015.
Wang, B. J., Lien, Y. H., and Yu, Z. R.: Supercritical fluid extractive fractionation-study of the antioxidant activities of propolis, Food Chem., 86, 237–243, 2003.
Yoshioka, T., Kawada, K., Shimada, T., and Mori, M.: Lipid peroxidation in maternal and cord blood and protective mechanism against activatedoxygen toxicity in the blood, Am. J. Obstet. Gynecol., 135, 372–376, 1979.
Zhuang, Y., Xing, C., Cao, H., Zhang, C., Luo, J., Guo, X., and Hu, G.: Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a highenergy low-protein diet, Sci. Rep., 9, 10141, https://doi.org/10.1038/s41598-019-46183-y, 2019.





