the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Lin28B overexpression decreases let-7b and let-7g levels and increases proliferation and estrogen secretion in Dolang sheep ovarian granulosa cells
Zhiyuan Sui
Yongjie Zhang
Zhishuai Zhang
Chenguang Wang
Xiaojun Li
Feng Xing
Although ovine puberty initiation has been previously studied, the mechanism by which the RNA-binding protein Lin28B affects this process has not been investigated. The present study aimed to investigate the effects of Lin28B overexpression on let-7b, let-7g, cell proliferation, and estrogen secretion in Dolang sheep ovine ovarian granulosa cells. In this study, a Lin28B vector was constructed and transfected into ovarian granulosa cells using liposomes. After 24, 48, and 72 h of overexpression, quantitative real-time PCR (qRT-PCR) was used for measuring let-7b and let-7g microRNA (miRNA) levels, and estrogen secretion was measured using the enzyme-linked immunosorbent assay (ELISA). A CCK-8 (Cell Counting Kit-8) kit was used for evaluating cell viability and proliferation in response to Lin28B overexpression at 24 h. The results showed that the expression of let-7b and let-7g decreased significantly after Lin28B overexpression, and the difference was consistent over different periods. The result of ELISA showed that estradiol (E2) levels significantly increased following Lin28B overexpression. Additionally, Lin28B overexpression significantly increased the cell viability and proliferation. Therefore, the Lin28B–let-7 family axis may play a key role in the initiation of female ovine puberty.
- Article
(3278 KB) - Full-text XML
- BibTeX
- EndNote
Puberty is the first estrus period in female animals during which ovulation occurs. Puberty is related to the hypothalamus–pituitary–gonadal axis regulation and the effect of environmental and genetic factors on the coordinating functions of luteinizing and follicle-stimulating hormones (Meeran et al., 2003; Redmond et al., 2011; Wankowska et al., 2008; Pool et al., 2020; Rosa and Bryant, 2003). Puberty is affected by various factors, including genetic mechanisms, nutrient levels, and light duration, all of which affect the timing of puberty (Greives et al., 2007; Suttie et al., 1985). Studies have shown that the Lin28B gene expression in the hypothalamus plays an important role in puberty initiation in mammals (Tommiska et al., 2010).
Lin28B is a highly conserved RNA-binding protein first discovered in Caenorhabditis elegans, and this heterochronic gene regulates nematode development from the larval to adult stages (Ambros, 1989). Lin28B was first cloned in human hepatocellular carcinoma. It is located on chromosome 6. Lin28B has a very long 3′-UTR (untranslated region) and a complementary site for let-7 microRNAs (Guo et al., 2006).
MicroRNAs (miRNAs), a type of endogenous non-coding small RNA composed of 20–30 nucleotides, are involved in cell development, proliferation, differentiation, and apoptosis by targeting specific mRNAs, mediating translational repression, or degrading mRNAs (Baek et al., 2008; Bartel, 2004; Hwang and Mendell, 2006). Lin28B inhibits the biogenesis of various miRNAs, including the let-7 family miRNAs. Let-7 miRNAs regulate genes related to cell growth and differentiation (Peng et al., 2011). Among them, let-7b inhibits cyclin D1 expression, which regulates the self-renewal of embryonic stem cells and the proliferation and tumorigenicity of cancer cells (Schultz et al., 2008; Xu et al., 2009; F. Yu et al., 2007). Lin28B suppresses miRNA let-7b expression to promote CD44+/Lin28B+ human pancreatic cancer stem cell proliferation and invasion (Shao et al., 2015).
In mammals, granulosa cell (GC) proliferation plays an important regulatory role in determining follicle fate and maturation (Douville and Sirard, 2014; Khan et al., 2016; Saatcioglu et al., 2016). GCs produce estradiol (E2), which supports their survival and proliferation and promotes follicle maturation (Chou and Chen, 2018). Mammalian follicle development is a key process within the ovary, to which GCs directly contribute through their proliferation and growth (Lv et al., 2019). In this context, previous reports indicate interactions among E2, Lin28B, and the let-7 family; 17-β-estradiol and let-7a–Lin28B axes synergistically affect the occurrence and development of adenomyosis (Huang et al., 2021). Additionally, treating MCF-7 cells with 17-β-estradiol (E2) resulted in rapid and specifically reduced let-7g expression (Qian et al., 2011).
Thus far, relatively few studies have reported on Lin28B and ovine puberty. In the present study, an overexpression vector was constructed from the Dolang sheep Lin28B sequence and transfected into sheep ovarian granulosa cells. This sequence was overexpressed for identifying its effects on let-7b, let-7g, cell proliferation, and estrogen secretion. Thus, this study lays a foundation for a better understanding of how Lin28B may contribute to the initiation of puberty in sheep.
2.1 Cell collection and culture
Ovaries of Dolang sheep (approximately 3.5 months old) were collected immediately after slaughter and placed in normal saline at 37 ∘C and brought back to the laboratory for processing. The cumulus–oocyte complex (COC) was aspirated from follicles with a diameter of 3–8 mm. GCs were collected after serial pipetting, and the follicular fluid and medium were mixed and injected into a sterile 15 mL centrifuge tube. The supernatant was discarded after centrifugation at 175 g for 5 min. Subsequently, 3 mL of DMEM (Dulbecco's Modified Eagle Medium) was added, stirred gently to mix, and centrifuged at 175 g for 5 min, and the supernatant was discarded. A freshly prepared complete medium (89 % DMEM + 10 % fetal bovine serum + 1 % penicillin streptomycin) (Gibco, USA) was added, mixed, and transferred to Petri dishes. Isolated ovarian granulosa cells were cultured at 37 ∘C under 5 % CO2 for subsequent experiments.
2.2 Immunofluorescence assay
Cell slides were placed into 24-well cell culture plates (2 × 104 cells per well), and 1 mL of culture medium was added. The cells were incubated for 24 h in an incubator. After the cells had attached to the slides, the medium was aspirated, and the cells were washed once with PBS (phosphate-buffered saline) (Gibco, USA), fixed with 4 % paraformaldehyde (Solarbio, Beijing, China) for 30 min at 4 ∘C, washed three times with PBS for 5 min each, and blocked with a blocking solution (0.5 % Triton X-100 mixed with PBS 1 : 1, plus 10 % goat serum) (Solarbio, Beijing, China) at room temperature for 2 h. The cell slides were incubated with a primary antibody (FSHR antibody : PBS = 1 : 100) (22665-1-AP, Proteintech, Wuhan, China) at 4 ∘C for 24 h. Subsequently, they were incubated with Goat anti-Rabbit IgG (H+L) Cross-Adsorbed (IgG : PBS = 1 : 500) (SA00006-3, Proteintech, Wuhan, China) for 2 h at room temperature in the dark and washed three times with PBS for 5 min each. The cells were stained with DAPI (DAPI : PBS = 1 : 1000) (Solarbio, Beijing, China) for 5 min and washed three times with PBS for 5 min each. One drop of Fluoromount-G (SouthernBiotech, USA) was dropped on the slide, and the side with cells was covered. Images were acquired using a fluorescence microscope (DS-Ri2; Nikon, Japan) and examined with a 100× objective.
2.3 Construction of Lin28B overexpression vector
The pEGFP-N1 vector was digested with NheI and EcoRI. The 754 bp coding sequence of Lin28B was amplified from the cDNA of Dolang sheep using qRT-PCR (Takara, Dalian, China) (Xing et al., 2019a). The amplified cDNA was digested with NheI and EcoRI and ligated into the pEGFP-N1 vector to generate pEGFP-N1–Lin28B, which was verified by DNA sequencing.

Figure 1Identification of sheep granulosa cells (GCs). (a) The red marker indicates cells expressing FSHR. (b) The blue marker indicates DAPI-stained nuclei. (c) Merge is a red fluorescently labeled FSHR with a blue fluorescently DAPI overlay. Bar: 100 µm.
2.4 Cell transfection
Cultured Dolang sheep ovary granulosa cells were suspended with PBS for later use. Trypan blue staining solution (0.4 %) was added to the cell suspension at a cell dye ratio of 1 : 1 (), and the cells were counted using a hemocytometer. According to the manufacturer's instructions for Lipofectamine 3000, 1×106 ovarian granulosa cells were plated in six-well plates and cultured for 24 h. The cells were transfected using a plasmid : transfection reagent ratio of 1 : 3. Cell transfection was divided into three periods: 24 h (0–24 h), 48 h (0–48 h), and 72 h (0–72 h). The cells were grouped as target gene, empty vector, and untreated cells. These experiments were run in triplicate. The medium from each time period was collected for measuring estrogen secretion, and cell RNA was extracted by TRIzol–chloroform extraction for quantitative real-time PCR (qRT-PCR).
2.5 Western blotting
The total cell protein was extracted using a kit (TransGen Biotech, China), and the protein concentration was detected using a BCA kit (TransGen Biotech). A 10 % separating gel and 5 % stacking gel were prepared for electrophoresis. The band was excised and transferred to the membrane, following which it was incubated overnight at 4 ∘C with the primary antibodies, namely anti-Lin28B (1 : 1000, Abcam, UK) and anti-ACTB (1 : 5000, Proteintech). The membrane was then incubated with an enzyme-conjugated secondary antibody for 2 h at 37 ∘C. The bands were detected using an ELC luminescence kit (Beyotime, Jiangsu, China).
2.6 Reverse transcription and expression of miRNA
The expression of Lin28B, let-7b, and let-7g in ovarian granulosa cells 24, 48, and 72 h after transfection was assessed using qRT-PCR.
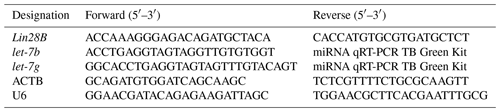
For Lin28B mRNA expression, ACTB was selected as the reference gene for normalizing mRNA levels. The primer sequences for Lin28B and ACTB mRNA are presented in Table 1.
For let-7b and let-7g quantification, cDNA was synthesized using a miRNA RT Kit (Takara, Dalian, China). The mature sequences of let-7b and let-7g were obtained using miRBase (https://www.mirbase.org/, last access: 18 April 2022) and were used to design the primers. The forward primers for let-7b and let-7g were designed, whereas the reverse primers were included in the Takara miRNA RT Kit. U6 was selected as a reference gene. Table 1 presents the primer sequences used for let-7b, let-7g, and U6 miRNA.
The qRT-PCR was performed using the Mir-X miRNA qRT-PCR TB Green Kit (Takara, Dalian, China). All experiments were run on an Eppendorf device (Eppendorf, Germany). Each sample and assay were run in triplicate. The levels of Lin28B, let-7b, and let-7g were calculated using the 2−ΔΔCT method.
2.7 Cell proliferation
Cell proliferation was measured using a CCK-8 (Cell Counting Kit-8) assay (Beyotime, Jiangsu, China). Cells (5×103) were resuspended in complete DMEM and seeded into a 96-well culture plate. The plate was incubated at 37 ∘C for 24 h. The incubator was maintained in a humidified atmosphere of 5 % CO2. WST-8 cell proliferation reagent was added, and the plate was incubated for an additional 2 h. Absorbance at 450 nm was measured using a microplate reader (BioTek, USA) to analyze the effect of Lin28B overexpression on cell proliferation.
2.8 Hormone determination
ELISA (Jining, Shanghai, China) was used for detecting E2 concentration in the cell culture media collected at 24, 48, and 72 h.
2.9 Statistical analysis
SPSS 26.0 software (IBM, Chicago, IL, USA) was used for statistical analysis. All experiments were repeated at least three times, and the experimental results are presented as the mean ± standard error. A t test was used for comparing two groups, and a one-way ANOVA was used for testing the differences among multiple groups. Statistical significance was set at P<0.05.
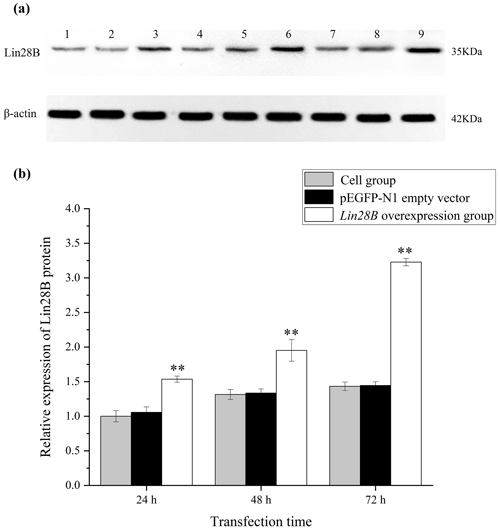
Figure 4Changes in Lin28B protein expression at 24, 48, and 72 h after transfection. P < 0.01. (a) Western blot strip diagram; (b) relative expression of Lin28B protein. Notes: (1) 24 h cell group; (2) 24 h empty-vector group; (3) 24 h Lin28B overexpression group; (4) 48 h cell group; (5) 48 h empty-vector group; (6) 48 h Lin28B overexpression group; (7) 72 h cell group; (8) 72 h empty-vector group; (9) 72 h Lin28B overexpression group.
3.1 Identification of ovine GCs
Through cell immunofluorescence identification (Fig. 1), this test showed that FSHR was expressed in ovarian granulosa cells, as indicated by red immunofluorescence; DAPI staining appeared as blue (Hong et al., 2022; Wang et al., 2022). Upon merging these images, the blue fluorescence of DAPI and the red fluorescence of FSHR completely overlapped, indicating that the cells are of high purity, meeting the requirements of subsequent experiments.
3.2 Expression of Lin28B in the granulosa cells of the ovaries
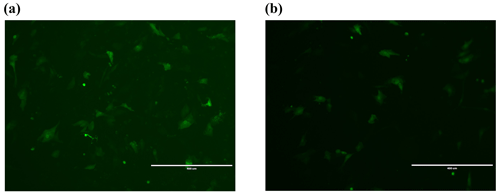
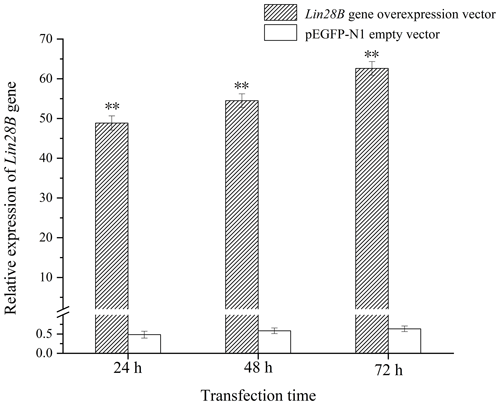
After transferring the Lin28B vector into ovarian granulosa cells, the fluorescence brightness of the test and control groups was examined at 24 h (Fig. 2), and Lin28B mRNA and protein expression levels were measured at 24, 48, and 72 h. After transfection, the mRNA (Fig. 3) and protein expression (Fig. 4) levels of the Lin28B gene in the overexpression group were significantly higher than those in the empty-vector group, indicating that the transfection was successful.
3.3 Effect of Lin28B overexpression on let-7b and let-7g
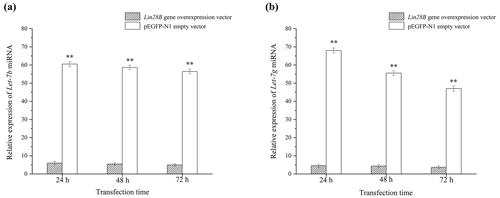
After cell transfection, the relative expression levels of let-7b and let-7g miRNA were measured using qRT-PCR (Fig. 5). The mRNA expression of the transfected Lin28B group was significantly higher than that of the empty-vector group (P<0.01), indicating that Lin28B was successfully overexpressed in the ovarian granulosa cells. After the cells were transfected, the expression levels of let-7b and let-7g in the empty-vector group were significantly higher than those in the overexpression group (P<0.01), which indicated that Lin28B overexpression significantly inhibited the expression of these miRNAs.
3.4 Effects of Lin28B overexpression on cell proliferation
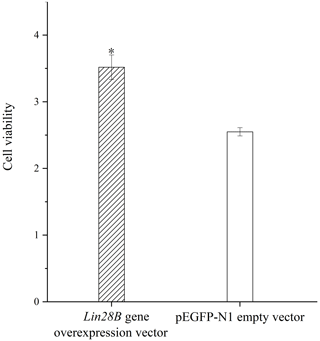
Granulosa cell proliferation was measured with and without Lin28B overexpression. The overexpression of Lin28B significantly increased ovarian granulosa cell proliferation compared with the empty-vector group (P<0.05; Fig. 6).
3.5 Effects of Lin28B overexpression on estradiol
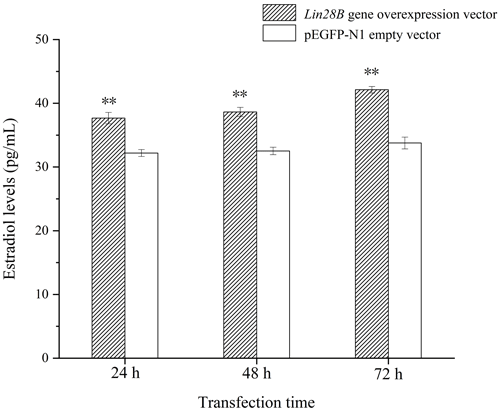
Following Lin28B overexpression, estradiol secretion by granulosa cells was measured using ELISA. The cell culture medium from cells overexpressing Lin28B for 24, 48, and 72 h was collected. The detection of estradiol is shown in Fig. 7. The concentrations at 24, 48, and 72 h in this group were significantly higher than those in the empty-vector group (P<0.05). Lin28B overexpression stably increased estradiol secretion at 24, 48, and 72 h. Estradiol levels in these periods were relatively stable, and the difference was not significant (P>0.05).
Puberty is the period when the reproductive ability of animals begins, and the length of puberty is related to the reproductive ability of animals (Xing et al., 2019b). Animals that reach puberty early have higher reproductive capacity and more offspring. Previous studies correlate puberty and Lin28B mRNA expression in livestock (Cao et al., 2020). To the best of our knowledge, this is the first study to investigate the role of Lin28B in sheep GCs. In the present study, an overexpression vector for Lin28B was constructed and transfected into GCs. The transfection was successful, as indicated by fluorescence imaging and relative mRNA expression. Following Lin28B overexpression, the let-7b and let-7g levels were found to be significantly lower than those of the control group. Let-7b and let-7g expression further decreased with increases in Lin28B expression. This result is consistent with Lin28B mRNA and let-7b miRNA expression in the hypothalamus of rats and female rhesus monkeys (Sangiao-Alvarellos et al., 2013).
Additionally, the present study showed that Lin28B overexpression promoted GC proliferation and E2 secretion. Other studies have shown similar results, even in cancer (Molenaar et al., 2012). According to related studies, the Lin28B–let-7 family axis regulates oncogenic cell function and the differentiation, growth, and metabolism of embryonic stem cells (Murray et al., 2013; Shyh-Chang and Daley, 2013). Aberrant expression of this axis is frequently observed in severe cancers, such as cervical, bladder, and lung cancers (Deng et al., 2017; Guo et al., 2017; Qi et al., 2018; Wu et al., 2019), and it normally regulates proliferative and metastatic functions. Furthermore, high Lin28B expression can promote the proliferation of human embryonic stem cells and accelerate their reprogramming process through faster cell division (Hanna et al., 2009; J. Yu et al., 2007). Let-7 miRNAs maintain differentiation patterns and normal development. Let-7 miRNAs and estrogen receptor (ER)α expressions are correlated (Sun et al., 2013). Estrogen secretion is suppressed by let-7 upregulation and decreases in ERα expression (Sun et al., 2016). The present study showed that Lin28B overexpression decreased let-7b and let-7g levels, promoted GC proliferation, and increased E2 secretion. Considering previous studies and our own, the Lin28B–let-7 family axis can be speculated to regulate GC proliferation and E2 secretion. However, the mechanism underlying this regulation should be investigated further.
In the present study, a vector overexpressing Lin28B was transfected into ovarian granulosa cells, whereby let-7b and let-7g miRNA levels decreased significantly. Concurrently, Lin28B overexpression can promote proliferation and estrogen secretion in sheep GCs. These findings can be used for further elucidating the regulatory mechanism of Lin28B in initiating sheep puberty.
No data sets were used in this article.
ZS: writing – original draft, conceptualization, methodology, data curation, visualization, investigation, software, writing review and editing. YZ: writing review and editing, data curation, supervision. ZZ: conceptualization, investigation. CW: conceptualization, methodology, editing. XL: data curation, software. FX: supervision, conceptualization, methodology, supervision, editing, funding acquisition, validation.
The contact author has declared that none of the authors has any competing interests.
For experimental animals, all protocols were performed in accordance with the “Guide to Animal Experimentation” and approved by the Use Committee under the norms of the Ethics Committee of Tarim University of Science and Technology (SYXK 2020-009).
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors thank all the facilities involved, including Tarim University and Xinjiang Production and Construction Corps.
This work was supported by the National Natural Science Foundation of China (grant no. 31960655).
This paper was edited by Joachim Weitzel and reviewed by two anonymous referees.
Ambros, V.: A hierarchy of regulatory genes controls a larva-to-adult developmental switch in C. elegans, Cell, 57, 49–57, https://doi.org/10.1016/0092-8674(89)90171-2, 1989.
Baek, D., Villen, J., Shin, C., Camargo, F. D., Gygi, S. P., and Bartel, D. P.: The impact of microRNAs on protein output, Nature, 455, 64–71, https://doi.org/10.1038/nature07242, 2008.
Bartel, D. P.: MicroRNAs: genomics, biogenesis, mechanism, and function, Cell, 116, 281–297, https://doi.org/10.1016/s0092-8674(04)00045-5, 2004.
Cao, G., Gao, Z., Jiang, Y., and Chu, M.: Lin28 gene and mammalian puberty, Mol. Reprod. Dev., 87, 525–533, https://doi.org/10.1002/mrd.23347, 2020.
Chou, C. H. and Chen, M. J.: The Effect of Steroid Hormones on Ovarian Follicle Development, Vitam. Horm., 107, 155–175, https://doi.org/10.1016/bs.vh.2018.01.013, 2018.
Deng, H. X., Yu, Y. Y., Zhou, A. Q., Zhu, J. L., Luo, L. N., Chen, W. Q., Hu, L., and Chen, G. X.: Yangzheng Sanjie decoction regulates proliferation and apoptosis of gastric cancer cells by enhancing let-7a expression, World J. Gastroentero., 23, 5538–5548, https://doi.org/10.3748/wjg.v23.i30.5538, 2017.
Douville, G. and Sirard, M. A.: Changes in granulosa cells gene expression associated with growth, plateau and atretic phases in medium bovine follicles, J. Ovarian. Res., 7, 50, https://doi.org/10.1186/1757-2215-7-50, 2014.
Greives, T. J., Mason, A. O., Scotti, M. A., Levine, J., Ketterson, E. D., Kriegsfeld, L. J., and Demas, G. E.: Environmental control of kisspeptin: implications for seasonal reproduction, Endocrinology, 148, 1158–1166, https://doi.org/10.1210/en.2006-1249, 2007.
Guo, M., Zhao, X., Yuan, X., Jiang, J., and Li, P.: MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer, Oncotarget, 8, 28226–28236, https://doi.org/10.18632/oncotarget.15999, 2017.
Guo, Y., Chen, Y., Ito, H., Watanabe, A., Ge, X., Kodama, T., and Aburatani, H.: Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma, Gene, 384, 51–61, https://doi.org/10.1016/j.gene.2006.07.011, 2006.
Hanna, J., Saha, K., Pando, B., Van Zon, J., Lengner, C. J., Creyghton, M. P., van Oudenaarden, A., and Jaenisch, R.: Direct cell reprogramming is a stochastic process amenable to acceleration, Nature, 462, 595–601, https://doi.org/10.1038/nature08592, 2009.
Hong, L., Chen, X., Zhu, M., Ao, Z., Tang, W., and Zhou, Z.: TIMP1 may affect goat prolificacy by regulating biological function of granulosa cells, Arch. Anim. Breed., 65, 105–111, https://doi.org/10.5194/aab-65-105-2022, 2022.
Huang, J. H., Duan, H., Wang, S., and Wang, Y. Y.: Estrogen 17betaestradiol accelerates the proliferation of uterine junctional zone smooth muscle cells via the let7a/Lin28B axis in adenomyosis, Mol. Med. Rep., 23, 337, https://doi.org/10.3892/mmr.2021.11976, 2021.
Hwang, H. W. and Mendell, J. T.: MicroRNAs in cell proliferation, cell death, and tumorigenesis, Br. J. Cancer, 94, 776–780, https://doi.org/10.1038/sj.bjc.6603023, 2006.
Khan, M. I., Dias, F. C., Dufort, I., Misra, V., Sirard, M. A., and Singh, J.: Stable reference genes in granulosa cells of bovine dominant follicles during follicular growth, FSH stimulation and maternal aging, Reprod. Fert. Develop., 28, 795–805, https://doi.org/10.1071/RD14089, 2016.
Lv, X., He, C., Huang, C., Wang, H., Hua, G., Wang, Z., Zhou, J., Chen, X., Ma, B., and Timm, B. K.: Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development, FASEB J., 33, 10049–10064, https://doi.org/10.1096/fj.201900179RR, 2019.
Meeran, D., Urbanski, H. F., Gregory, S. J., Townsend, J., and Tortonese, D. J.: Developmental changes in the hormonal identity of gonadotroph cells in the rhesus monkey pituitary gland, J. Clin. Endocrinol. Metab., 88, 2934–2942, https://doi.org/10.1210/jc.2002-021001, 2003.
Molenaar, J. J., Domingo-Fernandez, R., Ebus, M. E., Lindner, S., Koster, J., Drabek, K., Mestdagh, P., van Sluis, P., Valentijn, L. J., van Nes, J., Broekmans, M., Haneveld, F., Volckmann, R., Bray, I., Heukamp, L., Sprussel, A., Thor, T., Kieckbusch, K., Klein-Hitpass, L., Fischer, M., Vandesompele, J., Schramm, A., van Noesel, M. M., Varesio, L., Speleman, F., Eggert, A., Stallings, R. L., Caron, H. N., Versteeg, R., and Schulte, J. H.: LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression, Nat. Genet., 44, 1199–1206, https://doi.org/10.1038/ng.2436, 2012.
Murray, M. J., Saini, H. K., Siegler, C. A., Hanning, J. E., Barker, E. M., van Dongen, S., Ward, D. M., Raby, K. L., Groves, I. J., and Scarpini, C. G.: LIN28 Expression in Malignant Germ Cell Tumors Downregulates let-7 and Increases Oncogene LevelsLIN28/let-7 Deregulation in Malignant Germ Cell Tumors, Cancer Res., 73, 4872–4884, https://doi.org/10.1158/0008-5472.CAN-12-2085, 2013.
Peng, S., Chen, L.-L., Lei, X.-X., Yang, L., Lin, H., Carmichael, G. G., and Huang, Y.: Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells, Stem Cells, 29, 496–504, https://doi.org/10.1002/stem.591, 2011.
Pool, K. R., Rickard, J. P., and de Graaf, S. P.: Overcoming neuroendocrine and metabolic barriers to puberty: the role of melatonin in advancing puberty in ewe lambs, Domest. Anim. Endocrin., 72, 106457, https://doi.org/10.1016/j.domaniend.2020.106457, 2020.
Qi, L., Liu, F., Zhang, F., Zhang, S., Lv, L., Bi, Y., and Yu, Y.: lncRNA NEAT1 competes against let-7a to contribute to non-small cell lung cancer proliferation and metastasis, Biomed. Pharmacother., 103, 1507–1515, https://doi.org/10.1016/j.biopha.2018.04.053, 2018.
Qian, P., Zuo, Z., Wu, Z., Meng, X., Li, G., Wu, Z., Zhang, W., Tan, S., Pandey, V., Yao, Y., Wang, P., Zhao, L., Wang, J., Wu, Q., Song, E., Lobie, P. E., Yin, Z., and Zhu, T.: Pivotal role of reduced let-7g expression in breast cancer invasion and metastasis, Cancer Res., 71, 6463–6474, https://doi.org/10.1158/0008-5472.CAN-11-1322, 2011.
Redmond, J. S., Macedo, G. G., Velez, I. C., Caraty, A., Williams, G. L., and Amstalden, M.: Kisspeptin activates the hypothalamic-adenohypophyseal-gonadal axis in prepubertal ewe lambs, Reproduction, 141, 541–548, https://doi.org/10.1530/REP-10-0467, 2011.
Rosa, H. J. and Bryant, M. J.: Seasonality of reproduction in sheep, Small Ruminant Res., 48, 155–171, https://doi.org/10.1016/S0921-4488(03)00038-5, 2003.
Saatcioglu, H. D., Cuevas, I., and Castrillon, D. H.: Control of Oocyte Reawakening by Kit, PLoS Genet, 12, e1006215, https://doi.org/10.1371/journal.pgen.1006215, 2016.
Sangiao-Alvarellos, S., Manfredi-Lozano, M., Ruiz-Pino, F., Navarro, V. M., Sanchez-Garrido, M. A., Leon, S., Dieguez, C., Cordido, F., Matagne, V., Dissen, G. A., Ojeda, S. R., Pinilla, L., and Tena-Sempere, M.: Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty, Endocrinology, 154, 942–955, https://doi.org/10.1210/en.2012-2006, 2013.
Schultz, J., Lorenz, P., Gross, G., Ibrahim, S., and Kunz, M.: MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth, Cell Res., 18, 549–557, https://doi.org/10.1038/cr.2008.45, 2008.
Shao, Y., Zhang, L., Cui, L., Lou, W., Wang, D., Lu, W., Jin, D., and Liu, T.: LIN28B suppresses microRNA let-7b expression to promote CD44+/LIN28B+ human pancreatic cancer stem cell proliferation and invasion, Am. J. Cancer Res., 5, 2643–2659, PMID: 26609473; PMCID: PMC4633895, 2015.
Shyh-Chang, N. and Daley, G. Q.: Lin28: primal regulator of growth and metabolism in stem cells, Cell Stem. Cell, 12, 395–406, https://doi.org/10.1016/j.stem.2013.03.005, 2013.
Sun, X., Qin, S., Fan, C., Xu, C., Du, N., and Ren, H.: Let-7: a regulator of the ERalpha signaling pathway in human breast tumors and breast cancer stem cells, Oncol. Rep., 29, 2079–2087, https://doi.org/10.3892/or.2013.2330, 2013.
Sun, X., Xu, C., Tang, S. C., Wang, J., Wang, H., Wang, P., Du, N., Qin, S., Li, G., Xu, S., Tao, Z., Liu, D., and Ren, H.: Let-7c blocks estrogen-activated Wnt signaling in induction of self-renewal of breast cancer stem cells, Cancer Gene Ther., 23, 83–89, https://doi.org/10.1038/cgt.2016.3, 2016.
Suttie, J. M., Gluckman, P. D., Butler, J. H., Fennessy, P. F., Corson, I. D., and Laas, F. J.: Insulin-like growth factor 1 (IGF-1) antler-stimulating hormone?, Endocrinology, 116, 846–848, https://doi.org/10.1210/endo-116-2-846, 1985.
Tommiska, J., Wehkalampi, K., Vaaralahti, K., Laitinen, E. M., Raivio, T., and Dunkel, L.: LIN28B in constitutional delay of growth and puberty, J. Clin. Endocr. Metab., 95, 3063–3066, https://doi.org/10.1210/jc.2009-2344, 2010.
Wang, Y., Duan, C., Guo, Y., Li, J., He, H., Li, R., Zhang, Y., and Liu, Y.: Effects of glucose on glycolysis and steroidogenesis as well as related gene expression in ovine granulosa cells in vitro, Small Ruminant Res., 215, 106766, https://doi.org/10.1016/j.smallrumres.2022.106766, 2022.
Wankowska, M., Romanowicz, K., and Polkowska, J.: The neuroendocrine events during the ovine growth-promoted maturation: the developmental importance of hypophysiotrophic action of somatostatin in ewes, Anim. Reprod. Sci., 109, 146–160, https://doi.org/10.1016/j.anireprosci.2007.12.007, 2008.
Wu, J., Feng, X., Du, Y., Luan, B., Yu, H., Yu, Y., Wu, L., and Zhao, H.: beta-catenin/LIN28B promotes the proliferation of human choriocarcinoma cells via Let-7a repression, Acta Biochim. Biophys. Sin. (Shanghai), 51, 455–462, https://doi.org/10.1093/abbs/gmz027, 2019.
Xing, F., Zhang, C., and Kong, Z.: Cloning and expression of lin-28 homolog B gene in the onset of puberty in Duolang sheep, Asian-Austral. J. Anim., 32, 23–30, https://doi.org/10.5713/ajas.18.0276, 2019a.
Xing, F., Zhang, C., and Kong, Z.: Cloning and expression of lin-28 homolog B gene in the onset of puberty in Duolang sheep, Asian-Austral. J. Anim., 32, 23–30, https://doi.org/10.5713/ajas.18.0276, 2019b.
Xu, B., Zhang, K., and Huang, Y.: Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells, RNA, 15, 357–361, https://doi.org/10.1261/rna.1368009, 2009.
Yu, F., Yao, H., Zhu, P., Zhang, X., Pan, Q., Gong, C., Huang, Y., Hu, X., Su, F., Lieberman, J., and Song, E.: let-7 regulates self renewal and tumorigenicity of breast cancer cells, Cell, 131, 1109–1123, https://doi.org/10.1016/j.cell.2007.10.054, 2007.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., and Thomson, J. A.: Induced pluripotent stem cell lines derived from human somatic cells, Science, 318, 1917–1920, https://doi.org/10.1126/science.1151526, 2007.