the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Improved biological value of eggs due to the addition of pomegranate seed oil to laying-hen diets
Şaziye Canan Bölükbaşı
Büşra Dumlu
Aycan Mutlu Yağanoğlu
In this study, the effects of the addition of pomegranate seed oil (PSO) at different levels (0, 0.5, 1, and 1.5 mL kg−1) to laying-hen rations on performance values, egg quality criteria, egg shelf life, some enzyme activity, and the fatty acid composition of yolks were investigated. In the study, 96 Lohman LSL laying hens at 64 weeks of age were used. The trial consisted of four groups, each containing 24 hens. Chickens were given feed and water ad libitum during the 8-week experiment. The first group was the control group and was fed with a basal diet, while the other groups were fed with feeds with 0.5, 1.0, and 1.5 mL kg−1 PSO added to the basal feed, respectively.
The lowest feed consumption and the highest egg weight were determined in the 1 mL kg−1 PSO group. The highest feed conversion ratio, the lowest eggshell weight, and the shell-breaking strength were determined in the 0.5 mL kg−1 PSO group. It was determined that the egg yolk malondialdehyde (MDA) value in the groups to which pomegranate seed oil was added was significantly lower than the control group on the 28th day of storage. The lowest glutation (GSH) and catalase values were found in the control group, and the highest total antioxidant capacity (TAC) was found in the 1 mL kg−1 PSO group. It was determined that the addition of PSO to the diet significantly increased the rate of saturated fatty acids (SEFA), conjugated linoleic acid (CLA), and conjugated linolenic acids (CLnA) in yolk.
The results showed that the addition of 1 mL kg−1 pomegranate seed oil to the feeds decreased feed consumption, increased egg weight, and positively affected the shelf life of the egg. In short, the addition of PSO had a positive effect on shelf life, and it increased punicic acid and CLA levels without reducing egg quality.
- Article
(429 KB) - Full-text XML
- BibTeX
- EndNote
It is possible for people to maintain their vital activities with adequate and balanced nutrition, and animal foods have an important place in meeting the energy, protein, vitamins, and minerals required for adequate and balanced nutrition. Poultry breeding is an important option to fill this gap due to its significant advantages compared to other livestock branches. Among animal foods, eggs are an excellent food, as they are rich in nutrients, provide excellent quality protein, and are a great source of vitamin D (Applegate, 2000).
Today, consumers' tendency to choose functional foods, which are more beneficial for their physical and mental health, has led scientists to work in this field (Babicz-Zielińska, 2006). Therefore, to meet the need for animal products, efforts to increase product quality and obtain functional products continue intensively as does yield.
Some biologically active compounds are highly effective in preventing or treating chronic diseases, such as cardiovascular diseases, diabetes, and cancer. It is possible to enrich the egg with these biologically active compounds by changing the diet of chickens (Kostogrys et al., 2017). One of these biologically active components used in research is conjugated linoleic acid (CLA), which has many health benefits. However, there have been studies reporting that CLA added to the diet of laying hens has some negative effects on egg quality (Ahn et al., 1999; Kim et al., 2007; Franczyk-Zarow et al., 2008) as well as beneficial effects (Bölükbaşı and Erhan, 2005). Recently, there has been interest in conjugated linolenic acid (CLnA), due to its very important physiological properties. There are scientific studies on the antidiabetic, anticarcinogenic, hypocholesterolemic, and anti-obesity properties of CLnA (Hennessy et al., 2011; Shinohara et al., 2012b; Nekooeian et al., 2014). The inclusion of pomegranate seed oil (PSO) in a rat diet has been reported to significantly reduce blood triglyceride concentration (Białek et al., 2014). In a limited number of studies on this subject, it has been reported that CLnA added to laying-hen diets increases egg production and egg quality (Kostogrys et al., 2017; Ngo Njembe et al., 2021a, b).
One of the most important sources of CLnA is pomegranate. Pomegranate (Punica granatum L.) is native to Iran and the Himalayas in northern India, and it has been cultivated since ancient times throughout the Mediterranean region of Asia, Africa, and Europe (Morton and Dowling, 1987). In Türkiye, which ranks third in the world in terms of pomegranate production, approximately 537 000 t of pomegranate was produced in 2018, and approximately 30 % of this was exported, while the rest was used for domestic consumption (TUİK, 2018). Approximately 48 % of the total weight of a pomegranate consists of the peel (Zarei et al., 2011). The unused part of the seed and bark contains bioactive components rich in polyphenols (the peel contains 44.0 % and the seed is 02.6 % phenolics), vitamins, and polyunsaturated fatty acids (Singh et al., 2002; Seeram et al., 2005). It was reported that the addition of 4 % of pomegranate peel, which is rich in bioactive components, to broiler diets using waste oil reduced the peroxide value in meat (Sadabadi et al., 2021, 2022).
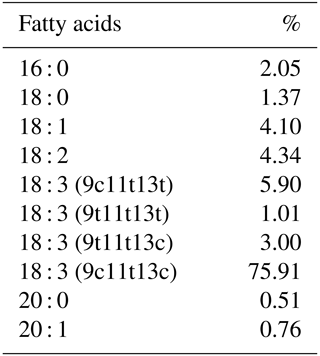
Pomegranate seed oil has anti-oxidant, anti-inflammatory, anticarcinogenic, hyperlipidemic, and antimicrobial effects. The pomegranate seed contains 12 %–20 % oil, and most of the oil it contains consists of conjugated octadecatrienoic fatty acid (CLnA). Punicic acid (cis 9, trans 11, cis 13 acid) is one of the most important isomers of CLnA with a share of 75 %–80 %.
This study aimed to enrich the egg in terms of CLnA (punicic acid) by adding pomegranate seed oil to laying-hen rations and to examine the effects of PSO on performance, egg quality criteria, egg shelf life, some enzymes, and egg yolk fatty acid composition.
2.1 Material
In this study, 96 Lohman LSL laying hens at 64 weeks of age were used. The trial consisted of four groups, each containing 24 hens (six subgroups). The first group was the control group and was fed with basal feed (Table 2), while the other groups were fed with feeds with 0.5, 1.0, and 1.5 mL kg−1 pomegranate seed oil added to the basal feed, respectively. Chickens were given feed and water ad libitum during the 8-week experiment.
The feeds used in the experiment were obtained from a commercial feed company. The organic pomegranate seed oil (containing 76 % punicic acid) used in the experiment was purchased from a commercial company (Table 1).
Table 2Composition of basal diet.
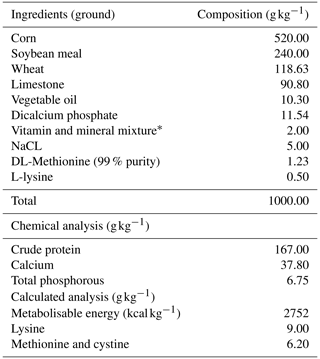
∗ Supplied per kilogram of diet: 12 000 IU vitamin A, 2500 IU vitamin D3, 30 IU vitamin E, 4 mg vitamin K3, 3 mg vitamin B1, 6 mg vitamin B2, 30 mg niacin, 10 mg calcium D-pantothenate, 5 mg vitamin B6, 0.015 mg vitamin B12, 1 mg folic acid, 0.050 mg D-biotin, 50 mg vitamin C, 300 mg choline chloride, 80 mg manganese, 60 mg iron, 60 mg zinc, 5 mg copper, 0.5 mg cobalt, 2 mg iodine, and 0.15 mg selenium.
2.2 Determination of general performance values
Feed consumption and egg production, egg weight, and damaged egg rate were determined by measurements made every 2 weeks. In addition, egg quality parameters such as shape index, shell thickness, breaking strength, white ratio, shell ratio, yolk ratio, and Haugh unit (Silversides, 1994) were determined in eggs collected from each subgroup (n=6).
2.3 Determination of egg yolk lipid oxidation amount
At the end of the experiment, eggs from each subgroup (n=6) were stored at +4 ∘C for thiobarbituric acid reactive substance (TBARS) analysis. The oxidation levels of the samples were measured on the 1st and 28th days of storage, according to the method determined by Tarladgis et al. (1960). After the egg yolk samples were placed in a Kjeldahl flask, distilled water and 6 N HCl were added and heated by steam distillation. Then, 5 mL aliquots were withdrawn and transferred to test tubes to which 5 mL of 2-TBA solution (0.02 mol L−1) were further added. These mixtures were kept in a water bath for 35 min. The mixtures, which were cooled under tap water for 5 min after being taken from the water bath, were read in the spectrophotometer at a wavelength of 538 nm. The TBARS values were described as milligrams malonaldehyde per kilogram of yolk.
2.4 Enzyme activities
After taking blood samples from each group (n=6) and centrifuging, the serum part was separated and kept at −20 ∘C until analysis. In the serum samples obtained, the activity measurement of glutathione peroxidase (glutathione assay kit, no. 703002, Cayman, USA, according to Matkovics et al., 1988), malondialdehyde (MDA) (glutathione assay kit, no. 10009055, Cayman, USA; according to Yoshioka et al., 1979), catalase (Goth, 1991), superoxide dismutase enzymes (SOD assay kit, no. 706002, Cayman, USA; according to Sun et al., 1988), total antioxidant capacity (Erel, 2004), and total oxidant capacity (Erel, 2005) were analyzed with commercial kits.
2.5 Lipid extraction and fatty acid analysis
Eggs from each subgroup (n=6) were stored at +4 ∘C for fatty acid composition. Egg yolk of 1 g was put into a 50 mL plastic tube. Isopropanol of 22.5 mL was added and shaken vigorously. Then, 12.5 mL of hexane was added to the tube, and the mixture was stirred for an additional 3 min. The mixture was centrifuged at 4000 rpm for 5 min: the upper phase was transferred to a flask, and the lower phase was washed twice with 12.5 mL of hexane solvent. Finally, the combined organic phases were dried with sodium sulfate (Na2SO4), and the solvent was removed in the evaporator at 30 ∘C (Tsiplakou et al., 2006).
The methylation process of the samples was performed according to Kelly et al. (1998) and was carried out within the framework of the protocol. Fatty acid methyl ester was prepared by transmethylation of egg yolk oil. Methyl acetate of 100 µL was added to a solution of 100 mg of oil in 1 mL of hexane and mixed. Then, 100 µL of methylation reactant (1.75 mL of methanol and 0.4 mL of sodium methylate aqueous solution of 5.4 M) was added to the mixture. The reaction mixture was stirred at room temperature for 10 min, and then 120 µL of hydrolysis solution (prepared by dissolving 1 g of oxalic acid in 30 mL of diethyl ether) was added to the reaction mixture to destroy excess sodium methylate. The mixture was centrifuged at 5 ∘C for 5 min, and the liquid was separated for chromatographic determination and used directly.
2.6 Determination of fatty acid composition
Chromatographic analyses were performed using a flame ionization detector (FID) on a Shimadzu gas chromatograph (GC) Autosystem. TR-CN100 Capillary Column (100 m×0.25 mm ID, 0.20 µm film) was used for analysis. The working principles of the GC-FID device were as follows: detector temperature of 250 ∘C, injector temperature of 240 ∘C, split model injection, split ratio of , helium carrier gas flow of 30.0 mL min−1 (constant flow model), hydrogen gas flow of 40 mL min−1, air flow of 400 mL min−1, and injection volume of 1.0 µL
2.7 Determination of diet chemical composition
Crude protein, calcium, and phosphorus contents of the diet were determined in accordance with AOAC (2002)
The differences between the groups were determined by analysis of variance (ANOVA) using the SPSS package program (SPSS 20.0). Duncan multiple comparison tests were used to compare the treatment averages.
The statistical model is as follows:
where Yij is the dependent variable, μ is the overall mean, ai is the effect of PSO, and eij is the random error.
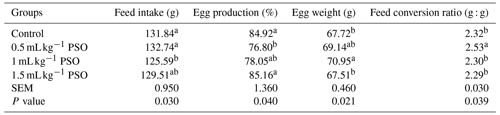
The effect of adding pomegranate seed oil to the rations on performance values is presented in Table 3. It was determined that the performance values were significantly affected by the addition of PSO. It was found that the addition of 1 mL kg−1 PSO to the ration significantly reduced feed consumption but increased egg weight. The lowest egg production and feed conversion rate were observed in the 0.5 mL kg−1 PSO group.
Table 3Effect of pomagranate seed oil on performance in laying hens.
a, b The column average is significantly different (p<0.05). SEM: standard error of the means.
In a study by Ngo Njembe et al. (2021a), it was determined that the addition of olive oil and flaxseed and pomegranate seed oil to the ration had no effect on egg production and egg weight. However, in another study by the same researchers, it was determined that the addition of 5 % PSO to the ration decreased feed consumption compared to the olive oil-added group but increased egg production significantly (Ngo Njembe et al., 2021b).
In their study, Kostogrys et al. (2017) added different levels of punicic acid to laying-hen rations, and they found that the feed consumption, egg production, and feed conversion ratio increased with an increase in punicic acid level. In a study conducted on laying hens, the effect of adding 5 %, 10 %, and 15 % pomegranate seed pulp to the ration on performance values was investigated. As a result of the research, it was determined that 5 % pomegranate pulp increased egg production and had no effect on feed consumption, feed efficiency, and egg weight. The researchers concluded that the increase in egg production was due to the presence of punicic acid in the pomegranate seed pulp (Saki et al., 2014). The number of studies examining the effect of pomegranate seed oil on laying hens is limited. Studies conducted to date have shown that PSO has a positive effect on egg production. In this study, it was determined that the addition of 0.5 mL kg−1 PSO significantly reduced egg production compared to the control group. This may be due to the fact that the level of punicic acid in the diet of the chickens in this group is lower than in the other groups, so the chickens in this group adapt to the diet later and have less bio-availability from the feed.
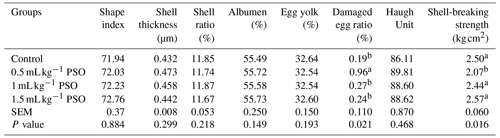
It was observed that the addition of PSO to the diet had no effect on shape index, shell thickness, albumen ratio, shell ratio, egg yolk, and Haugh unit (Table 4). It was found that the rate of damaged eggs and shell-breaking strength were affected by PSO. It was determined that the addition of 0.5 mL kg−1 PSO to the diet significantly decreased the rate of damaged eggs and the shell-breaking strength (P<0.05). In addition to studies reporting that dietary CLA levels improve egg quality (Bölükbaşı and Erhan, 2005), there are also studies that report the negative effects on egg quality such as the Hough unit, shell-breaking strength, and egg hardness (Kim et al., 2007; Franczyk-Zarow et al., 2008). In this study, the damaged egg ratio and shell-breaking strength were adversely affected only in the 0.5 mL kg−1 PSO group. This may be due to the fact that groups with a higher percentage of PSO have high CLnA values as well as CLA, as there are studies reporting that CLnA improves egg quality (Kostogrys et al., 2017). Kim et al. (2007) reported that the use of CLA in combination with several other fatty acids improved egg quality. Saki et al. (2014) reported that adding different levels of pomegranate seed pulp to the ration had no effect on egg quality criteria.
Table 4Effect of pomagranate seed oil on egg quality in laying hens.
a, b The column average is significantly different (p<0.05). SEM: standard error of the means.
There was no significant difference between the groups in terms of TBARS values on the 1st day of storage. However, at the end of the 4-week storage period (28th day), it was determined that the TBARS value was significantly lower (P<0.01) in the groups that had added PSO to the diet compared to the control group (Table 5). The decrease in the TBARS value was found to be associated with the increase in the egg yolk of CLA and CLnA which have antioxidant effects. There are studies showing that the TBARS value in egg yolk and chicken meat decreases depending on the increase in the CLA level in the diet (Bölükbaşı and Erhan, 2005, 2007; Bölükbaşı, 2006).
Table 5Effect of PSO on TBARS of egg values of yolk in laying hens (mg MDA kg−1).
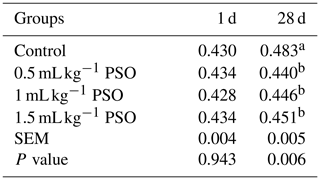
a, b The column average is significantly different. SEM: standard error of the means.
Rojo-Gutiérrez et al. (2021) determined that pomegranate seed oil showed 91.29 % scavenging activity against DPPH radicals. In this study, it was determined that punicic acid in pomegranate seed oil accumulated in egg yolk at a significant level. Therefore, the TBARS values of egg yolks were found to be lower on the 28th day compared to the control group.
It has been shown in many studies that the antioxidant activity of pomegranate peels, seeds, and juice is quite high (Gil et al., 2000; Rojo-Gutiérrez et al., 2021; Singh et al., 2002; Li et al., 2006; Shabtay et al., 2008). Devatkal and Naveena (2010) observed that the TBARS value decreased significantly in their study in which they examined the effect on the shelf life of goat meat by adding 2 % pomegranate seed powder. In addition, there are many studies showing that processing chicken and red meat with pomegranate juice increases the shelf life (Naveena et al., 2008; Vaithiyanathan et al., 2011; Devatkal and Naveena, 2010).
The addition of pomegranate seed oil to the laying-hen diet was found to significantly alter serum enzymes (Table 6). It was determined that glutation (GSH) and catalase values were significantly higher (P<0.01) in the PSO groups compared to the control group. The highest GSH was found in the 1.5 mL kg−1 PSO group, and the highest catalase values were detected in the 0.5 and 1 mL kg−1 PSO groups. It was found that the MDA value was significantly higher (P<0.01) in the 1 and 1.5 mL kg−1 PSO groups. It was observed that the addition of 1.5 mL kg−1 PSO to the ration significantly reduced the serum SOD value (P<0.01). The highest TAS (total antioxidant) value was found in the 1 mL kg−1 PSO group, and the lowest TOS (total oxidant) value was found in the 0.5 mL kg−1 PSO group.
Table 6Effects of PSO on some antioxidant enzymes in the serum of the laying hens.
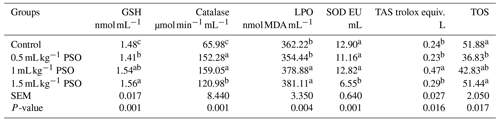
a, b, c The column average is significantly different. SEM: standard error of the means.
Saki et al. (2014) found that the addition of 5 % pomegranate seed meal to laying-hen rations had no effect on total serum antioxidants but increased the MDA value. The researchers have suggested that pomegranate seed oil contains a high amount of polyunsaturated fatty acids (PUFAs), long-chain fatty acids that are more sensitive to oxidation, and that the plasma MDA level may therefore be increased. In a study on rats, it was reported that 50 mg kg−1 of pomegranate peel extract added to the ration significantly protected catalase, peroxidase and SOD enzyme activities (Chidambara Murthy et al., 2002). Similarly, Saha and Ghosh (2009) reported that punicic acid increased the activity of catalase, glutathione and SOD in rats.
In this study, it was determined that the TAS value was quite low in the 1 mL kg−1 PSO group. Rojo-Gutiérrez et al. (2021) reported that pomegranate seed oil showed 91.29 % scavenging activity against DPPH radical. The reason for the decrease in TAS value can be explained by the antioxidant property of PSO.
The addition of PSO to the diet had no effect on myristic, palmitic, oleic, linoleic, gamma linolenic, alpha linolenic, arachidonic, docosahexaenoic acid (DHA), and monounsaturated fatty acids (MUFA) (Table 7). It was observed that the addition of PSO decreased the palmitioleic acid ratio and increased the stearic acid, CLA, punicic acid, eicosapentaenoic acid (EPA), SFA, and PUFA. The highest stearic acid and SFA values were found in the 1 mL kg−1 PSO group, while the highest CLA, CLnA (punicic acid), and PUFA values were found in the 1.5 mL kg−1 PSO group.
Table 7Effects of PSO on the fatty acid composition in the yolk of laying hens.
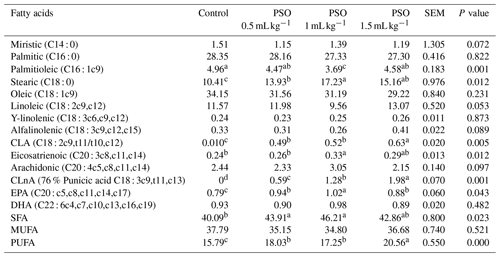
a, b, c, d The raw average is significantly different. SEM: standard error of the means.
In this study, it was observed that the addition of PSO to the ration increased EPA and the CLA ratio, and an increase in the SEFA ratio was observed in parallel with the increase in the CLA ratio. Ngo Njembe et al. (2021b) reported that the addition of PSO to the laying-hen diets decreased the MUFA ratio statistically significantly, while the SFA ratio was numerically higher than the control group. Trans 10 cis 12 CLA isomer decreases MUFA and increases SFA and sterol coenzyme A desaturase enzyme activity in the liver. This enzyme catalyzes the double bond between the c9 and c10 atoms of palmitioleic and oleic fatty acids, which convert to 16 : 0 and 18 : 0 (Smith et al., 2002). Similar to this study, Kostogrys et al. (2017) reported that the ratio of SFA and CLA increased and MUFA ratio decreased with the increase of punicic acid level in laying-hen diets. Stearoyl-CoA desaturase (SCD) is an enzyme that catalyzes the Δ9-cis desaturation of fatty acyl-CoA substrates and converts palmitoyl- and stearoyl-CoA to palmitoleoyl- and oleoyl-CoA, respectively. Several studies have shown that CLnA inhibits Δ9 desaturation in the liver through inhibition of stearoyl-CoA desaturase activity and reduction of SCD-1 mRNA expression (Arao et al., 2004; Shinohara et al., 2012a). In the present study, it was determined that the CLnA level increased with the increase PSO level in the diet, and it is thought that increasing CLnA in the diet containing high PSO inhibits the SCD enzyme, and accordingly, MUFA decreases and SFA increases. Many researchers have suggested that the fatty acid profile of egg yolks is affected by dietary fatty acid composition (Bölükbaşı et al., 2006; Kostogrys et al., 2017).
Pomegranate seed oil is very rich in polyunsaturated fatty acid, about 55 %–78 % of which is punicic acid (cis 9, trans 11, cis 13 acid). The CLnA (containing 76 % punicic acid) ratio in egg yolk increased with the increase of the PSO ratio in the diet in this study. Similarly, it has been reported that the addition of different levels of punicic acid (Kostogrys et al., 2017) to the diet increases the rate of punicic acid in egg yolks. Ngo Njembe et al. (2021a) reported that the addition of PSO to laying-hen rations increased the ratio of CLA and punicic acid. The authors speculated that the inclusion of PSO in the laying-hen diet also allowed some degree of accumulation of another isomer of CLA (t9,t11-CLA), possibly derived from the metabolism of CLnA, apart from the punicic acid found in PSO. Ngo Njembe et al. (2021a) show that laying hens can efficiently transform various CLnA isomers with a D13 double bond. In several previous studies, it has been reported that CLnA isomers (punicic acid, α-eleostearic acid) can be converted to rumenic acid (t9, t11-CLA) via a Δ13 reductase in animal and human tissues (Suzuki et al., 2006; Schneider et al., 2013).
It has been reported that egg quality decreased in some studies carried out to increase CLA and CLnA levels in eggs by adding chicken meal, fish meal, fish oil, and flaxseed to rations (Hayat et al., 2010; Dunn-Horrocks et al., 2011). However, in a limited number of studies on PSO, it has been reported that the amount of CLA and CLnA in the egg yolk will be increased without reducing egg quality (Kostogrys et al., 2017).
In conclusion, it was observed that the addition of PSO to rations decreased the feed consumption, especially in the 1 mL kg−1 PSO group, and it increased the egg weight in this study. It was determined that the shelf life of eggs was positively affected in all groups to which PSO was added. As a result, it was found that the shelf life was positively affected and the levels of CLnA (punicic acid) and CLA increased in eggs without reducing egg quality with the addition of PSO.
The data presented in this study are available free of charge for any user upon reasonable request from the corresponding author.
ŞCB, BD, and AMY designed the study, AMY performed the statistical analysis, and ŞCB, BD, and AMY wrote the paper.
The contact author has declared that none of the authors has any competing interests.
The research protocol was approved and applied in accordance with the Animal Ethics Committee Guidelines of Atatürk University (no. 2016/78).
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was supported by Atatürk University (project no. 2016/269).
This research was supported by Atatürk University (project no. 2016/269).
This paper was edited by Manfred Mielenz and reviewed by Mohammad Ghasemi-Sadabadi and one anonymous referee.
Ahn, D. U., Sell, J. L., Jo, C., Chamruspollert, M., and Jeffrey, M.: Effect of dietary conjugated linoleic acid on the quality characteristics of chicken eggs during refrigerated storage, Poultry Sci., 78, 922–928, 1999.
AOAC: Official Methods of Analysis of Association of Official Analytical Chemists, 17th Edn., edited by: Gaithersburg, M. D., AOAC, Washington, DC, ISBN 978-093558467-7, 2002.
Applegate, E.: Nutritional and functional roles of eggs in the diet, J. Am. Coll. Nutr., 19, 495–498, 2000.
Arao, K., Wang, Y. M., Inoue, N., Hirata, J., Cha, J.-Y., Nagao, K., and Yanagita, T.: Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats, Lipids Health Dis., 3, 24, https://doi.org/10.1186/1476-511X-3-24, 2004.
Babicz-Zielińska, E.: Role of psychological factors in food choice, Pol. J. Food Nutr. Sci., 56, 379–384, 2006.
Bialek, A., Jelinska, M., Bamburowicz-Klimkowska, M., and Tokarz, A.: Effect of bitter melon aqueous extract and pomegranate oil on glucose concentration and lipid profile in blood of rats – preliminary study, Int. J. Cardiol. Lipidol. Res., 1, 1–7, https://doi.org/10.15379/2410-2822.2014.01.01.01, 2014.
Bölükbaşı, Ş. C.: Effect of dietary conjugated linoleic acid (CLA) on broiler performance, serum lipoprotein levels, fatty acid composition in muscles and meat quality during refrigerated storage, Brit. Poultry Sci., 47, 470–479, 2006.
Bölükbaşı, Ş. C. and Erhan, M. K.: Effect of dietary conjugated linoleic acid on laying hen performance, egg yolk fatty acid composition and egg quality during refrigerated storage, J. Anim. Feed Sci., 14, 685–693, 2005.
Bölükbaşı, Ş. C. and Erhan, M. K.: Effects of semi replacement of dietary olive oil and corn oil with conjugated linoleic acid (CLA) on broiler performance, serum lipoprotein levels, fatty acid composition in muscles and meat quality during refrigerated storage, J. Anim. Vet. Adv., 6, 262–266, 2007.
Bölükbaşı, Ş. C., Erhan, M. K., and Özkan, A.: Effect of dietary thyme oil and vitamin E on growth, lipid oxidation, meat fatty acid composition and serum lipoproteins of broilers, S. Afr. J. Anim. Sci., 36, 189–196, 2006.
Chidambara Murthy, K. N., Jayaprakasha, G. K., and Ravendra, P. S.: Studies on antioxidant activity of pomegranate (Punica granatum L.) peel extract using in vivo models, J. Agr. Food Chem., 50, 4791–4795, 2002.
Devatkal, S. K. and Naveena, B. M.: Effect of salt, kinnow and pomegranate fruit by-product powders on color and oxidative stability of raw ground goat meat during refrigerated storage, Meat Sci., 85, 306–311, 2010.
Dunn-Horrocks, S., Pichardo-Fuchs, M., Lee, J., Ruiz-Feria, C., Creger, C., Hyatt, D., Stringfellow, K., Sanchez, M., and Farnell, M.: Effect of omega-3 enriched layer rations on egg quality, Int. J. Poult. Sci., 10, 8–11, 2011.
Erel, O.: A novel automated method to measure total antioxidant response against potent free radical reactions, Clin. Biochem., 37, 112–119, https://doi.org/10.1016/j.clinbiochem.2003.10.014, 2004.
Erel, O.: A new automated colorimetric method for measuring total oxidant status, Clin. Biochem., 38, 1103–1111, https://doi.org/10.1016/j.clinbiochem.2005.08.008, 2005.
Franczyk-Zarow, M., Kostogrys, R. B., Szymczyk, B., Jawien, J., Gajda, M., Cichocki, T., Wojnar, L., Chlopicki, S., and Pisulewski, P. M.: Functional effects of eggs, naturally enriched with conjugated linoleic acid, on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice(apoE/LDLR/), Brit. J. Nutr., 99, 49–58, 2008.
Gil, M. I., Tomás-Barberán, F. A., Hess-Pierce, B., Holcroft, D. M., and Kader, A. A.: Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing, J. Agr. Food Chem., 48, 4581–4589, 2000.
Goth, L.: A simple method for determenation of serum catalase activity and revision of serum catalase activity and revision of reference range, Clin. Chim. Acta, 196, 143–152, 1991.
Hayat, Z., Cherian, G., Pasha, T. N., Khattak, F. M., and Jabbar, M. A.: Sensory evaluation and consumer acceptance of eggs from hens fed flax seed and 2 different antioxidants, Poult. Sci. J., 89, 2293–2298, 2010.
Hennessy, A. A., Ross, R. P., Devery, R., and Stanton, C.: The health promoting properties of the conjugated isomers of α-linolenic acid, Lipids, 46, 105–119, https://doi.org/10.1007/s11745-010-3501-5, 2011.
Kelly, M. L., Kolver, E. S., Bauman, D. E., VanAmburgh, M. E., and Muller, L. D.: Effect of intake of pasture on con centrations of conjugated linoleic acid in milk of lactating cows, Int. J. Dairy Sci., 81, 1630–1636, 1998.
Kim, J. H., Hwangbo, J., Choi, N. J., Park, H. G., Yoon, D. H., Park, E. W., Lee, S. H., Park, B. K., and Kim, Y. J.: Effect of dietary supplementation with conjugated linoleic acid, with oleic, linoleic, or linolenic acid, on egg quality characteristics and fat accumulation in the egg yolk, Poultry Sci., 86, 1180–1186, 2007.
Kostogrys, R. B., Filipiak-Florkiewicz, A., Dereń, K., Drahun, A., Czyżyńska-Cichoń, I., Cieslik, E., Szymczyk, B., and Franczyk-Zarów, M.: Effect of dietary pomegranate seed oil on laying hen performance and physicochemical properties of eggs, Food Chem., 221, 1096–1103, 2017.
Li, Y., Guo, C., Yang, J., Wei, J., Xu, J., and Cheng, S.: Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract, Food Chem., 96, 254–260, 2006.
Matkovics, B., Szabo, L., and Varga, I. S.: Determination of enzyme activities in lipid peroxidation and glutathione pathways, Laboratoriumi Diagnosztika, 15, 248–249, 1988.
Morton, J. F. and Dowling, J. F.: Pomegranate: Fruits of warm climates, Miami University, Miami, USA, 352–355, ISBN 0961018410, 1987.
Nekooeian, A. A., Eftekhari, M. H., Adibi, S., and Rajaeifard, A.: Effect of pomegranate seed oil on insulin release in rats with type 2 diabetes, Iran. J. Med. Sci., 39, 130–135, 2014.
Ngo Njembe, M. T., Dejonghe, L., Verstraelen, E., Mignolet, E., Leclercq, M., Dailly, H., Gardin, C., Buchet, M., Waingeh Nain, C., and Larondelle, Y.: The egg yolk content in ω-3 and conjugated fatty acids can be sustainably increased upon long-term feeding of laying hens with a diet containing flaxseeds and pomegranate seed oil, Foods, 10, 1134, https://doi.org/10.3390/foods10051134, 2021a.
Ngo Njembe, M. T., Dormal, E., Gardin, C., Mignolet, E., Debier, C., and Larondelle, Y.: Effect of the dietary combination of flaxseed and Ricinodendron heudelotii or Punica granatum seed oil on the fatty acid profile of eggs, Food Chem., 344, 128668, https://doi.org/10.1016/j.foodchem.2020.128668, 2021b.
Rojo-Gutiérrez, E., Carrasco-Molinar, O., Tirado-Gallegos, J. M., Levario-Gómez, A., Chávez-González, M. L., Baeza-Jiménez, R., and Buenrostro-Figueroa, J. J.: Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil, J. Food Meas. Charact., 15, 2098–2107, https://doi.org/10.1007/s11694-020-00804-7, 2021.
Sadabadi, M. G., Ebrahimnezhad, Y., Sis, N. M., Teli, A. S., Ghalehkandi, J. G., and Veldkamp, T.: Supplementation of pomegranate processing waste and waste soybean cooking oil as an alternative feed resource with vitamin E in broiler nutrition: effects on productive performance, meat quality and meat fatty acid composition, Arch. Anim. Nutr., 75, 355–375, https://doi.org/10.1080/1745039X.2021.1965414, 2021.
Sadabadi, M. G., Ebrahimnezhad, Y., Sis, N. M., Teli, A. S., Ghalehkandi, J. G., and Veldkamp, T.: Effects of supplementation of pomegranate processing by-products and waste cooking oils as alternative feed resources in broiler nutrition, Sci. Rep., 12, 21216, https://doi.org/10.1038/s41598-022-25761-7, 2022.
Saha, S. S. and Ghosh, M.: Comparative study of antioxidant activity of α-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite, Food Chem. Toxicol., 47, 2551–2556, https://doi.org/10.1016/j.fct.2009.07.012, 2009.
Saki, A. A., Rabet, M., Zamani, P., and Yousefi, A.: The effects of different levels of pomegranate seed pulp with multi-enzyme on performance, egg quality and serum antioxidant in laying hens, Iran. J. Appl. Anim. Sci., 4, 803–808, 2014.
Schneider, A. C., Mignolet, E., Schneider, Y. J., and Larondelle, Y.: Uptake of conjugated linolenic acids and conversion to cis-9, trans-11-or trans-9, trans-11-conjugated linoleic acids in Caco-2 cells, Brit. J. Nutr., 109, 57–64, 2013.
Seeram, N. P., Adams, L. S., Henning, S. M., Niu, Y., Zhang, Y., Nair, M. G., and Heber, D.: In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice, J. Nutr. Biochem., 16, 360–367, 2005.
Shabtay, A., Eitam, H., Tadmor, Y., Orlov, A., Meir, A., Weinberg, P., Weinberg, Z. G., Chen, Y., Brosh, A., Izhaki, I., and Kerem, Z.: Nutritive and antioxidative potential of fresh and stored pomegranate industrial by-product as a novel beef cattle feed, J. Agr. Food Chem., 56, 10063–10070, 2008.
Shinohara, N., Ito, J., Tsuduki, T., Honma, T., Kijima, R., Sugawara, S., and Yokoyama, M.: Jacaric acid, a linolenic acid isomer with a conjugated triene system, reduces stearoyl-CoA desaturase expression in liver of mice, J. Oleo Sci., 61, 433–441, https://doi.org/10.5650/jos.61.433, 2012a.
Shinohara, N., Tsuduki, T., Ito, J., Honma, T., Kijima, R., Sugawara, S., Arai, T., Yamasaki, M., Ikezaki, A., Yokoyama, M., Nishiyama, K., Nakagawa, K., Miyazawa, T., and Ikeda, I.: Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo, BBA Mol. Cell Biol. L., 1821, 980–988, https://doi.org/10.1016/j.bbalip.2012.04.001, 2012b.
Silversides, F. G.: The Haugh unit correction for egg weight is not adequate for comparing eggs from chickens of different lines and ages, J. Appl. Poultry Res., 3, 120–126, 1994.
Singh, R. P., Chidambara Murthy, K. N., and Jayaprakasha, G. K.: Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models, J. Agr. Food Chem., 50, 81–86, 2002.
Smith, S. B., Hively, T. S., Cortese, G. M., Han, J. J., Chung, K. Y., Casteñada, P., Gilbert, C. D., Adams, V. L., and Mersmann, H. J.: Conjugated linoleic acid depresses the Δ9 desaturase index and stearoyl coenzyme A desaturase enzyme activity in porcine subcutaneous adipose tissue, J. Anim. Sci., 80, 2110–2115, 2002.
Sun, Y., Oberley, L. W., and Li, Y.: A simple method for clinical assay of superoxide dismutase, Clin. Chem., 34, 497–500, 1988.
Suzuki, R., Yasui, Y., Kohno, H., Miyamoto, S., Hosokawa, M., Miyashita, K., and Tanaka, T.: Catalpa seed oil rich in 9t, 11t, 13c-conjugated linolenic acid suppresses the development of colonic aberrant crypt foci induced by azoxymethane in rats, Oncol. Rep., 16, 989–996, 2006.
Tarladgis, B. G., Watts, B. M., Younathan, M. T., and Dugan, L.: Distillation method for the quantitative determination of malonaldehyde in rancid foods, J. Am. Oil Chem. Soc., 37, 44–48, 1960.
Tsiplakou, E., Mountzouris, K. C., and Zervas, G.: The effect of breed, stage of lactation and parity on sheep milk fat CLA content under the same feeding practices, Livest. Sci., 105, 162–167, 2006.
Türkiye İstatistik Kurumu (TÜİK): Meyveler içecek ve baharat bitkileri, https://biruni.tuik.gov.tr/medas/?kn=80&locale=tr (last access: 10 May 2019), 2018 (in Turkish).
Vaithiyanathan, S., Naveena, B. M., Muthukumar, M., Girish, P. S., and Kondaiah, N.: Effect of dipping in pomegranate (Punica granatum) fruit juice phenolic solution on the shelf life of chicken meat under refrigerated storage (4 ∘C), Meat Sci., 88, 409–414, 2011.
Yoshioka, T., Kawada, K., Shimada, T., and Mori, M.: Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood, Am. J. Obstet. Gynecol., 135, 372–376, 1979.
Zarei, M., Azizi, M., and Bashir-Sadr, Z.: Evaluation of physicochemical characteristics of pomegranate (Punica granatum L.) fruit during ripening, Fruits, 66, 121–129, 2011.