the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Novel alternative splicing variants of ACOX1 and their differential expression patterns in goats
Xian-Feng Wu
Yuan Liu
Cheng-Fang Gao
Xin-Zhu Chen
Xiao-Pei Zhang
Wen-Yang Li
As the first and rate-limiting enzyme of the peroxisomal β-oxidation pathway, acyl-coenzyme A oxidase 1 (ACOX1), which is regulated by peroxisome proliferator-activated alfa (PPARα), is vital for fatty acid oxidation and deposition, especially in the lipid metabolism of very long-chain fatty acids. Alternative splicing events of ACOX1 have been detected in rodents, Nile tilapia, zebra fish and humans but not in goats. Herein, we identified a novel splice variant of the ACOX1 gene, which was designated as ACOX1-SV1, in addition to the complete transcript, ACOX1, in goats. The length of the ACOX1-SV1 coding sequence was 1983 bp, which presented a novel exon 2 variation owing to alternative 5′-splice site selection in exon 2 and partial intron 1, compared to that in ACOX1. The protein sequence analysis indicated that ACOX1-SV1 was conserved across different species. Reverse-transcription quantitative real-time polymerase chain reaction (RT-qPCR) analysis showed that these two isoforms were expressed spatially and differently in different tissue types. ACOX1 and ACOX1-SV1 were expressed at high levels in liver, spleen, brain and adipose tissue in kid goats, and they were abundantly expressed in the fat, liver and spleen of adults. Interestingly, whether in kids or in adults, in fat, the mRNA level of ACOX1 was considerably higher than that of ACOX1-SV1. In contrast, in the liver, the expression of ACOX1-SV1 was considerably higher than that of ACOX1. This differential expression patterns showed the existence of a tissue-dependent splice regulation. These novel findings for ACOX1 should provide new insights for further studies on the function of ACOX1 and its variants that should aid in the breeding of goats with improved meat quality.
- Article
(12807 KB) - Full-text XML
-
Supplement
(2165 KB) - BibTeX
- EndNote
Goat meat is expected to assume greater importance in the future owing to the rapidly growing world population and a steady increase in the consumption of this meat (Henchion et al., 2014); moreover, meat with better qualities and improved flavor is desirable and demanded by consumers. The Fuqing goat breed is one of the finest local breeds found on the east coast of China and provides high-quality meat that is tender and has only a slight odor. Nonetheless, research on goat breeding for improving the quality of meat has lagged behind that on pork and beef. Data on candidate genes that affect the performance and quality of meat have largely been lacking, warranting detailed studies in this direction.
The peroxisomal fatty acid β-oxidation pathway, which is an essential metabolic pathway that operates in all eukaryotic cells (Kunau et al., 1995; Shi et al., 2016), is the main metabolic destination of very long-chain fatty acids (VLCFAs). This pathway plays a key role in the infants of mammals, wherein lipids in the breast milk become the major source of energy (Ehara et al., 2015). Importantly, this pathway is regulated directly by the peroxisome proliferator-activated receptor alfa (PPARα), which is a master transcriptional regulator of the fatty acid metabolism (Poirier et al., 2006).
Acyl-coenzyme A oxidase 1 (ACOX1) is the first and the rate-limiting enzyme of the peroxisomal β-oxidation pathway. It is mainly activated in peroxisome proliferators (a kind of organelle in the liver) and oxidizes long and medium straight-chain fatty acid substrates (Poirier et al., 2006; Wang et al., 2014). A deficiency of this enzyme leads to severe microvesicular steatohepatitis in mice (Fan et al., 1998; Yu et al., 2003). The lack of both ACOX1 and PPARα causes the absence of spontaneous hepatic peroxisome proliferation in aged mice (Hashimoto et al., 1999; Tanaka et al., 2017), highlighting the close connection between ACOX1 and PPARα. In humans, the absence of ACOX1 is associated with pseudoneonatal adrenoleukodystrophy (P-NALD), which is a disorder of the peroxisomes (El Hajj et al., 2012). The ACOX1 gene holds great promise as a potential target for improving meat performance in pig and cattle. For instance, an A/C polymorphism in intron 9 was found to be significantly associated with fat deposition in pig (Zuo et al., 2007), and the other one (A1865C) in exon 13 was significantly related to back fat thickness and marbling score in cattle, as evidenced from ultrasound imaging (Jiao et al., 2011). These observations point to the critical role of ACOX1 in the fatty acid metabolism and suggest its potential functions in affecting the development and deposition of fat.
Alternative splicing (AS), which exists in almost all kinds of mammals (Kelemen et al., 2013), is a fundamental molecular event that results in the production of different mature transcripts from the same primary RNA sequence (Sammeth et al., 2008). These transcripts contribute to diverse biological processes, such as cell differentiation (Kianianmomeni et al., 2014; Cotter et al., 2015), disease development (Sohail et al., 2014) and the adipocyte metabolism (Fiszbein et al., 2017). Previous studies reported that the splicing variants of PPARG (PPARG1 and PPARG2) could affect the lipogenesis of mammary tissue via the upregulated expressions of key lipogenic gene networks (Shi et al., 2014). The different transcripts of the SFRS18 gene (SFRS18_V1 and SFRS18_V2) were revealed to correlate with intramuscular fat content in pigs (Wang et al., 2009). Thus, AS may affect meat quality by regulating the expressions of key genes within the myocyte and adipocyte metabolism, especially the intramuscular lipocyte generation. Splicing variants of the ACOX1 gene were detected in Nile tilapia (He et al., 2014), mice (Vluggens et al., 2010) and humans (Oaxaca-Castillo et al., 2007); however, they have not been reported in goats. These studies all lend credence to the notion that the ACOX1 gene plays fundamental roles in meat quality. Therefore, this study aims to explore potential splicing transcripts of the goat ACOX1 gene and the tissue expression pattern in kid and adult goats, which would be of benefit for further study on the function of the ACOX1 gene and breeding programs in goats.
Experimental animals and procedures used in this study were approved by the International Animal Care and Use Committee of the Fujian Academy of Agricultural Sciences (FAAS), Fujian province, China. The care and use of experimental animals fully complied with local animal welfare laws, guidelines and policies.
2.1 Animals and tissue collection
Seven Fuqing goats (four adult wethers and three female kids (lactation period)) were fed and slaughtered in the goat breeding farm of FAAS. Because the development of gonads in goats does not begin during the lactation period, the experiments would not be influenced by gender. Nine types of tissue were collected for the study: heart, liver, spleen, lung, kidney, leg muscle, the longissimus muscle, perirenal fat and brain. All the samples were snap-frozen in liquid nitrogen and subsequently stored at −80 ∘C.
2.2 Total RNA isolation, first-strand cDNA synthesis and cDNA pool construction
Total RNA was isolated from the collected tissue samples using an RNA extraction kit and RNase-free DNase I (TaKaRa, Dalian, China). The genomic DNA was removed by treatment with RNase-free DNase I, and the quality of RNA was determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE) and 1 % agarose gel electrophoresis (Li et al., 2013). Reverse-transcription polymerase chain reaction (RT-PCR) was performed to synthesize complementary DNA (cDNA) using a PrimeScript™ RT reagent Kit (TaKaRa), according to the manufacturer's recommended procedure (S. H. Zhang et al., 2016).
2.3 Identification of ACOX1 alternative splice variants
The primers were designed, based on the predicted goat ACOX1 mRNA sequence (XM_013972412.1), using Primer Premier 5 software (Premier BioSoft, Palo Alto, CA, USA); the sequences of the primers are shown in Table 1. A pair of primers (P1) was designed for the identification of different splice transcripts in the goat ACOX1 gene (Table 1). The polymerase chain reactions (PCRs) were performed as described in our previous study (X. Y. Zhang et al., 2015).
2.4 Clone sequencing and validation of ACOX1 transcript variants
The PCR products were separated by electrophoresis on agarose gel and were cloned into a pGEM-T easy vector (Promega, Shanghai, China) after purification. The recombinant vector thus obtained was then transferred to Escherichia coli DH5α competent cells (TaKaRa) and sequenced (X. Y. Zhang et al., 2016).
2.5 Bioinformatics analysis
DNA and amino acid sequences were aligned using MEGA 5.1 (http://www.megasoftware.net/) and BioXM 2.6 (Nanjing Agricultural University). Phylogenetic trees were constructed using the neighbor-joining method implemented in MEGA (Lee et al., 2014) and by using NCBI pairwise alignments (http://www.ncbi.nlm.nih.gov/blast).
2.6 Measurement of ACOX1 and ACOX1-SV1 mRNA levels using quantitative real-time PCR
The specific primers used for reverse-transcription quantitative real-time polymerase chain reaction (RT-qPCR) were shown in Table 1. RT-qPCR was performed with Eastep® qPCR Master Mix Kit (Promega) in an Eppendorf Mastercycler ep Realplex 4 Real-Time PCR system. The RT-qPCR reaction system was implemented as described in our previous study (X. Y. Zhang et al., 2015). The steps in RT-qPCR were as follows: denaturation at 95 ∘C for 30 s, followed by 39 cycles of 15 s at 95 ∘C, and 50 s at 63 ∘C. The individual samples were amplified in triplicates, and a no-template control (NTC) was included (which contained ddH2O instead of cDNA template).
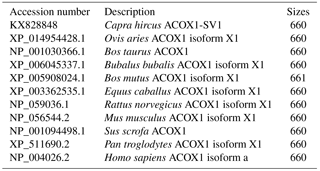
Figure 1Detection of different isoforms (ACOX1, ACOX1-SV1) of ACOX1 in goats and schematic of genomic structure. (a) Electrophoresis of the products obtained by RT-PCR of the mixed cDNA pool; (b) goat ACOX1 (XM_013972412) was used as the reference sequence in the diagram. Note: basic exons are shown in white boxes and alternatively spliced intron regions are shown in black box. Lines represent the basic introns. Positions of the primers P2 and P3 for RT-qPCR of the ACOX1 and the ACOX1-SV1 transcript variants.
2.7 Statistical analysis
The popular 2 method was used to calculate the variations in the expression levels; GAPDH was used as the reference gene (Livak et al., 2001). The reactions in which water was used instead of the template were regarded as NTCs. The calculation in the 2 method was performed using the following formula: ratio = 2, where (Bustin et al., 2009). Nine tissue samples were used for the two variants. Three individuals were used for each tissue and different isoforms were detected from the same individual for each tissue. Statistical differences between the expression levels of the different ACOX1 isoforms were analyzed by Student's t test using the SPSS 18.0 software. The values with uppercase letters indicate statistical significance at P<0.01 and those with lowercase letters indicate statistical significance at P<0.05.

Figure 3Sequence alignment of ACOX1 transcript variants from goat and human. (a) ACOX1 XM_013972412 is aligned with human ACOX1b NM_004035.6; (b) ACOX1-SV1 KX828848 is aligned with human ACOX1a NM_007292.5). Note: the gray uppercase letters represent the sequence of the novel exon 2 variation event in ACOX1-SV1. Boxes represent the initiation and termination codons.
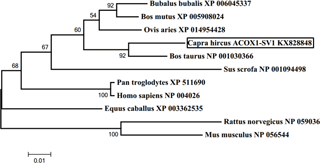
Figure 4Phylogenetic tree of ACOX1-SV1 made by MEGA 5.1. Note: the tree was constructed based on amino acid similarities of the full mRNA sequences by MEGA 5.1. The accession numbers and description of the sequences used for the construction of the tree were shown in Table 2. The numbers on the joints were bootstrap test values, and the branch length represents the evolutionary time.
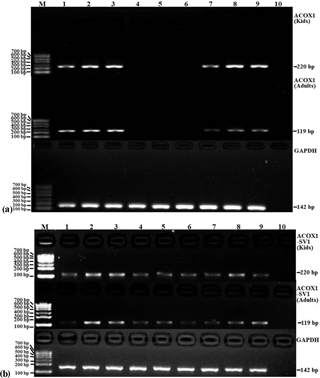
Figure 5The mRNA expression patterns of ACOX1 (a), ACOX1-SV1 (b) and GAPDH from various tissue types in the groups of kids and adults. Note: lanes (Ln) 1 – heart; Ln 2 – liver; Ln 3 – spleen; Ln 4 – lung; Ln 5 – kidney; Ln 6 – leg muscle; Ln 7 – longissimus muscle; Ln 8 – adipose; Ln 9 – brain; Ln 10 – NTC. Marker: marker I.
3.1 Identification of a novel transcript of the ACOX1 gene
The cDNAs prepared from the samples obtained from the kids and adults were pooled and the ACOX1 CDS was amplified using Primer P1 (Fig. 1a). The amplified products were cloned and sequenced. We detected the presence of the full-length CDS of two ACOX1 isoforms: the known ACOX1 transcript (XM_013972412.1) and a novel variant, named ACOX1-SV1 (GenBank Accession number: KX828848). The coding sequences of both ACOX1 and ACOX1-SV1 were 1983 bp in length (Fig. 2a), comprising 13 exons. However, ACOX1-SV1 was different from the ACOX1 transcripts, which had a novel exon 2 variation owing to an alternative 5′ splice site selection of exon 2 and partial intron 1 (NC_022311.1 nt4020–4120) (Fig. 1b).
3.2 Bioinformatics analysis of ACOX1 and ACOX1-SV1
The alignment of the ACOX1 and ACOX1-SV1 sequences (having the same number of amino acids) revealed a 40-amino acid (aa) difference (Fig. 2b). The amino acid sequence of goat ACOX1-SV1 was observed to share 95, 94, 92, 91, 91, 88, 88, 86, 85 and 82 % similarity with the corresponding sequences from Bos taurus (NP_001030366.1), Ovis aries (XP_014954428.1), Bubalus bubalis (XP_006045337.1), Bos mutus (XP_005908024.1), Equus caballus (XP_003362535.1), Pan troglodytes (XP_511690.2), Homo sapiens (NP_004026.2), Sus scrofa (NP_001094498.1), Rattus norvegicus (NP_059036.1) and Mus musculus (NP_056544.2). In the Basic Local Alignment Search Tool (BLAST) search using the CDS of goat isoforms as queries, we detected a high degree of similarity of the goat ACOX1 (XM_013972412) with the functional human counterpart, ACOX1b (NM_004035.6), and that of ACOX1-SV1 (KX828848) with human ACOX1a (NM_007292.5) (Fig. 3a and b) (Vluggens et al., 2010). Moreover, the splice site of goat 121-nucleotide sequence in ACOX1-SV1 was highly similar (99.2 %) to that of human ACOX1a isoform.
A phylogenetic tree was constructed based on the ACOX1-SV1 sequence for studying evolutionary relationships. It was observed that the goat ACOX1-SV1 was conserved in different species; it was closest to B. taurus ACOX1 and most distant from the clade comprising sequences from R. norvegicus and M. musculus (Fig. 4).
3.3 Relative and differential expression of ACOX1 and ACOX1-SV1 in different tissue types from different goat groups
The expression profiles of ACOX1 and ACOX1-SV1 were determined using semiquantitative RT-PCR in both the kids and adults. ACOX1 was observed to be expressed only in six tissue types, namely heart, liver, spleen, the longissimus muscle, fat and brain (Fig. 5a). However, the ACOX1-SV1 isoform was detected in all of the collected tissue (heart, liver, spleen, lung, kidney, leg muscle, longissimus muscle, fat and brain) (Fig. 5b).
Figure 6Melt peak (a) and melt curve (b) chart of ACOX1, ACOX1-SV1 and the reference gene GAPDH for RT-qPCR.
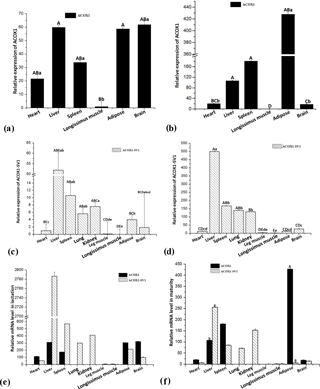
Figure 7Relative ACOX1 and ACOX1-SV1 mRNA levels in the kids (a, c) and adults (b, d), and differential expression levels of two different transcripts in various tissue types of kids (e) and adults (f). Note: gene expression was normalized using GAPDH as a reference gene. Uppercase letters represent statistical significance at P<0.01, and lowercase letters represent the statistical significance at P<0.05.
RT-qPCR was performed to determine the mRNA levels of the two isoforms in the experimental tissue samples (ACOX1: six tissue samples; ACOX1-SV1: nine tissue samples) collected from the two groups (Fig. 6). For kid goats, the expression of ACOX1 in the longissimus muscle was the lowest (P<0.05) (Fig. 7a). The mRNA level of ACOX-SV1 was significantly higher in liver, spleen, kidney and lung samples than in the other tissue types (P<0.05), followed by adipose tissue, the brain and the heart. Moreover, its expression in the leg muscle and longissimus muscle was low (Fig. 7c). In the adults, the ACOX1 expression level was the highest in adipose tissue; it was much higher in the spleen and liver compared to the levels in the heart, brain and longissimus muscle (P<0.01) (Fig. 7b). The expression of the ACOX1-SV1 isoform was highest in the liver, followed by that in the spleen, lung and kidney. Moreover, the expression of ACOX1-SV1 in the brain, adipose tissue, the heart, the leg and the longissimus muscle was also low (Fig. 7d). Overall, in kids, the two isoforms had a relatively higher expression in the liver, the spleen, the brain and adipose tissue and displayed low levels of expression in the heart and longissimus muscle. In adult individuals, the expression of the two isoforms was totally different in liver and adipose tissue; the mRNA level of ACOX1 was much higher than that of ACOX1-SV1 (P=0.001), whereas in the liver the expression of ACOX1-SV1 was higher than that of ACOX1 (P=0.02). Both the transcripts are expressed moderately in the spleen and weakly in the heart, brain and longissimus muscle.
As a major mechanism for the expansion of transcriptome and proteome diversity (Bush et al., 2017), AS is a principal contributor to the evolution of phenotypic complexity in mammals. This type of evolution was also demonstrated for the ACOX1 gene, a rate-limiting and relatively conserved enzyme in the peroxisomal fatty β-oxidation pathway. Several studies revealed similar sequences in other vertebrates, such as rodents, Nile tilapia, zebra fish and humans (Iyazawa et al., 1987; Morais et al., 2007; He et al., 2014). The ACOX1 transcripts were abundant in liver, adipose and kidney tissue. In this study, a novel splice variant, ACOX1-SV1 (KX828848), was first identified in goats; this variant was remarkably conserved in different species. Sequence alignment illustrated that the sequence of ACOX1-SV1 was highly similar to that of its human functional isoform, ACOX1a (Fig. 3b) (Vluggens et al., 2010), and ACOX1 was homologous to human ACOX1b (Fig. 3a). Splice variants of human ACOX1 (ACOX1a and 1b) were reported to be critical regulators in the fatty acid metabolism (Varanasi et al., 1994; Baes and Van Veldhoven, 2012), indicating that these variants were essential for the lipid metabolism in goats.
Interestingly, both the ACOX1 and ACOX1-SV1 variants were expressed spaciously and differently in the collected tissue samples. The mRNA levels of ACOX1 and ACOX1-SV1 were extremely different in the liver and fat, respectively. In liver tissue, ACOX1 had a moderate expression level, and the expression of ACOX1-SV1 was excellent in kid and adult goats, being 1.5 times higher than that of ACOX1 (Fig. 7e and f). Based on the results in humans, ACOX1 and ACOX1-SV1 might work as a dimer. Furthermore, the proteins encoded by these two isoforms should have critical functions in reducing PPARα activation and should subsequently affect the fatty acid metabolism in goats. The human ACOX1b isoform (compared to the goat ACOX1) reversed the ACOX1 null mice fatty liver phenotype (Oaxaca-Castillo et al., 2007). We, therefore, speculated that goat ACOX1 was the primary transcript used in the hepatic lipid metabolism of VLCFAs. Accordingly, ACOX1 and ACOX1-SV1 isoforms have a high probability of being the key regulators of the metabolism of VLCFAs in goats.
The ACOX1 isoform had high expression levels in fat when goats became mature. In contrast, the ACOX1-SV1 mRNA was rarely expressed in both the kid and adult individuals, suggesting that the ACOX1 isoform not only played a key role in the hepatic fatty acid metabolism but might also greatly affect the adipocyte differentiation and deposition of fat in goats. From the published reports, ACOX1 proteins are known to regulate adipose differentiation and are implicated in obesity (Baranova et al., 2013). They are used as markers of lipogenesis for RT-qPCR (Palou et al., 2009; Flachs et al., 2005). Furthermore, the expression level of ACOX in adults was considerably higher than that in kids, which could be explained by adipose deposition leading to a peak in expression after the individuals mature. In contrast, ACOX1-SV1 is likely to be involved in the hepatic fatty acid metabolism. The differential expression of ACOX1 and ACOX1-SV1 shows the existence of tissue-dependent splice regulation, which allowed for evolutionary selection (Kelemen et al., 2013).
The mRNA levels were found to change in the brain. In kid goat, ACOX1 and ACOX1-SV1 were abundantly expressed in the brain, whereas their expression was decreased in adults. Beyond their role in the lipid metabolism, ACOX1 and ACOX1-SV1 are involved in the synthesis of docosahexaenoic acid (DHA), which is best represented polyunsaturated fatty acid (PUFA) in brain and nervous tissue and is critical for the extension of neuronal axons and in neuronal processes (Trompier et al., 2014). When ACOX1 is mutated, the plasma level of DHA is reduced (Baes and Van Veldhoven, 2012) and the neurological development of the infant dramatically deteriorates (Masson et al., 2016). The suggested transcripts of the ACOX1 gene were thus crucial in the development of the brain in kid goats.
In conclusion, two alternative splice variants of ACOX1 (ACOX1 and ACOX1-SV1) were identified in kid and adult goats, and they were found to express at high levels in the liver, spleen, brain and fat tissue of kids. In adult individuals, the two isoforms were expressed at high levels in fat, the liver and the spleen. In both the cases lipid conversion and deposition were observed to be important factors involved in meat performance. These findings would provide a foundation for further investigation of the functions of ACOX1 and its variants in goat breeding, especially in the improvement of meat quality.
The splice variant (ACOX1-SV1) that we have found has been uploaded to the National Center for Biotechnology Information (NCBI) Nucleotide Database (GenBank, accession number: KX828848), available at: https://www.ncbi.nlm.nih.gov/nuccore/KX828848 (Wu, 2017). The sequencing data of ACOX1-SV1 are provided in the Supplement.
| ACOX | acyl-coenzyme A oxidase |
| ACOX1 | acyl-coenzyme A oxidase 1 |
| ACOX1-SV1 | acyl-coenzyme A oxidase 1 splice variant 1 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| PPARα | peroxisome proliferator-activated receptor alfa |
| P-NALD | pseudoneonatal adrenoleukodystrophy |
| VLCFAs | very long-chain fatty acids |
| AS | alternative splicing |
| mRNA | messenger RNA |
| CDS | coding sequence |
| cDNA | complementary DNA |
| PCR | polymerase chain reaction |
| RT-PCR | reverse-transcription polymerase chain reaction |
| RT-qPCR | reverse-transcription quantitative real-time polymerase chain reaction |
| aa | amino acid |
| bp | base pair |
| Cq | quantification cycle |
| SD | standard deviation |
| NCBI | National Center for Biotechnology Information |
| BLAST | Basic Local Alignment Search Tool |
| DHA | docosahexaenoic acid |
| PUFA | polyunsaturated fatty acid |
The supplement related to this article is available online at: https://doi.org/10.5194/aab-61-59-2018-supplement.
The authors declare that they have no conflict of interest.
This work was supported by the Public Research Project of Fujian province
(2016R1022-8), the Young Talent Innovation Foundation of the Fujian Academy
of Agricultural Sciences (2015QC-3), and the Young Talent Fund of the
Institute of Animal Husbandry and Veterinary Medicine (MYQJ2015-4). We are
deeply thankful to the staff of the goat breeding farm, Fuzhou, Fujian
province, PR China, for collecting the samples.
Edited by: Steffen Maak
Reviewed by: Mingxun
Li and one anonymous referee
Baes, M. and Van Veldhoven, P. P.: Mouse models for peroxisome biogenesis defects and β-oxidation enzyme deficiencies, BBA-Mol. Basis Dis., 1822, 1489–1500, 2012.
Baranova, A., Tran, T. P., Afendy, A., Wang, L., Shamsaddini, A., Mehta, R., Chandhoke, V., Birerdinc, A., and Younossi, Z. M.: Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS), J. Transl. Med., 11, 133, https://doi.org/10.1186/1479-5876-11-133, 2013.
Bush, S. J., Chen, L., Tovar-Corona, J. M., and Urrutia, A. O.: Alternative splicing and the evolution of phenotypic novelty, Philos. T. R. Soc. B., 372, 20150474, https://doi.org/10.1098/rstb.2015.0474, 2017.
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., Mueller, R., Nolan, T., Pfaffl, M. W., and Shipley, G. L.: The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments, Clin. Chem., 55, 611–622, 2009.
Cotter, K. A., Nacci, D., Champlin, D., Chuprin, J., and Callard, G. V.: Cloning of multiple ERα mRNA variants in killifish (Fundulus heteroclitus), and differential expression by tissue type, stage of reproduction, and estrogen exposure in fish from polluted and unpolluted environments, Aquat. Toxicol., 159, 184–197, 2015.
Ehara, T., Kamei, Y., Yuan, X., Takahashi, M., Kanai, S., Tamura, E., Tsujimoto, K., Tamiya, T., Nakagawa, Y., and Shimano, H.: Ligand-activated PPARα-dependent DNA demethylation regulates the fatty acid β-oxidation genes in the postnatal liver, Diabetes, 64, 775–784, 2015.
El Hajj, H. I., Vluggens, A., Andreoletti, P., Ragot, K., Mandard, S., and Kersten, S.: The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy), Endocrino., 53, 2568–2575, 2012.
Fan, C. Y., Pan, J., Usuda, N., Yeldandi, A. V., Rao, M. S., and Reddy, J. K.: Steatohepatitis, Spontaneous Peroxisome Proliferation and Liver Tumors in Mice Lacking Peroxisomal Fatty Acyl-CoA Oxidase Implications for Peroxisome Proliferator-Activated Receptor α Natural Ligand Metabolism, J. Biol. Chem., 273, 15639–15645, 1998.
Fiszbein, A. and Kornblihtt, A. R.: Alternative splicing switches: Important players in cell differentiation, BioEssays, 39, 1600157, https://doi.org/10.1002/bies.201600157, 2017.
Flachs, P., Horakova, O., Brauner, P., Rossmeisl, M., Pecina, P., Franssen-van Hal, N., Ruzickova, J., Sponarova, J., Drahota, Z., Vlcek, C., Keijer, J., Houstek, J., and Kopecky, J.: Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce β-oxidation in white fat, Diabetologia, 48, 2365–2375, 2005.
Hashimoto, T., Fujita, T., Usuda, N., Cook, W., Qi, C., and Peters, J. M.: Peroxisomal and mitochondrial fatty acid β-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor α and peroxisomal fatty acyl-coA oxidase genotype correlation with fatty liver phenotype, J. Biol. Chem., 274, 19228–19236, 1999.
He, A.-Y., Liu, C.-Z., Chen, L.-Q., Ning, L.-J., Zhang, M.-L., Li, E.-C., and Du, Z.-Y.: Identification, characterization and nutritional regulation of two isoforms of acyl-coenzyme A oxidase 1 gene in Nile tilapia (Oreochromis niloticus), Gene, 545, 30–35, 2014.
Henchion, M., McCarthy, M., Resconi, V. C., and Troy, D.: Meat consumption: Trends and quality matters, Meat Sci., 98, 561–568, 2014.
Iyazawa, S., Ayashi, H., Hijikata, M., Shii, N., Furuta, S., Kagamiyama, H., Osumi, T., and Hashimoto, T.: Complete nucleotide sequence of cDNA and predicted amino acid sequence of rat acyl-CoA oxidase, J. Biol. Chem., 262, 8131–8137, 1987.
Jiao, Y., Zan, L. S., Liu, Y. F., and Wang, H. B.: Molecular characterization, polymorphism of the ACOX1 gene and association with ultrasound traits in Bos Taurus, Genet. Mol. Res., 10, 1948–1957, 2011.
Kelemen, O., Convertini, P., Zhang, Z., Wen, Y., Shen, M., Falaleeva, M., and Stamm, S.: Function of alternative splicing, Gene, 514, 1–30, 2013.
Kianianmomeni, A., Ong, C. S., Rätsch, G., and Hallmann, A.: Genome-wide analysis of alternative splicing in Volvox carteri, BMC genomics, 15, 1117, 2014.
Kunau, W. H., Dommes V., and Schulz, H.: β-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress, Progress in lipid research, Prog. Lipid Res., 34, 267–342, 1995.
Lee, T. H., Guo, H., Wangm, X., Kim, C., and Paterson, A. H.: SNPhylo: a pipeline to construct a phylogenetic tree from huge SNP data, BMC Genomics, 15, 162, https://doi.org/10.1186/1471-2164-15-162, 2014.
Li, M. X., Sun, X. M., Hua, L. S., Huang, Y. Z., Wang, J., Cao, X. K., Zhan, Z. Y., Lan, X. Y., and Chen, H.: Molecular characterization, alternative splicing and expression analysis of bovine DBC1, Gene, 527, 689–693, 2013.
Livak, K. J. and Schmittgen, T. D.: Analysis of relative gene expression data using realtime quantitative PCR and the 2(−delta delta C(T)) method, Methods, 25, 402–408, 2001.
Masson, R., Guerra, S., Cerini, R., Pensato, V., Gellera, C., Taroni, F., and Simonati, A.: Early white matter involvement in an infant carrying a novel mutation in ACOX1, Eur. J. Paediatr. Neuro., 20, 431–434, 2016.
Morais, S., Knoll-Gellida, A., André, M., Barthe, C., and Babin, P. J.: Conserved expression of alternative splicing variants of peroxisomal acyl-CoA oxidase 1 in vertebrates and developmental and nutritional regulation in fish, Physiol. Genomics, 28, 239–252, 2007.
Oaxaca-Castillo, D., Andreoletti, P., Vluggens, A., Yu, S., Van Veldhoven, P. P., Reddy, J. K., and Cherkaoui-Malki, M.: Biochemical characterization of two functional human liver acyl-CoA oxidase isoforms 1a and 1b encoded by a single gene, Bioph. Res. Co., 360, 314–319, 2007.
Palou, M., Priego, T., Sánchez, J., Rodríguez, A. M., Palou, A., and Picó, C.: Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features, Cell. Physiol. Biochem., 24, 547–556, 2009.
Poirier, Y., Antonenkov, V. D., Glumoff, T., and Hiltunen, J. K.: Peroxisomal β-oxidation-a metabolic pathway with multiple functions, BBA-Mol. Cell Res., 1763, 1413–1426, 2006,
Sammeth, M., Foissac, S., and Guigó, R.: A general definition and nomenclature for alternative splicing events, PLoS Comput. Biol., 4, e1000147, https://doi.org/10.1371/journal.pcbi.1000147, 2008.
Shi, H. B., Zhao, W. S., Luo, J., Yao, D. W., Sun, Y. T., Li, J., Shi, H. P., and Loor, J. J.: Peroxisome proliferator-activated receptor γ1 and γ2 isoforms alter lipogenic gene networks in goat mammary epithelial cells to different extents, J. Dairy Sci., 97, 5437–5447, 2014.
Shi, Y., Sun, X., Sun, Y., Hou, L., Yao, M., Lian, K., Li, J., Lu, X., and Jiang, L.: Elevation of cortical C26: 0 due to the decline of peroxisomal β-oxidation potentiates amyloid β generation and spatial memory deficits via oxidative stress in diabetic rats, Neuroscience, 315, 125–135, 2016.
Sohail, M., Cao, W., Mahmood, N., Myschyshyn, M., Hong, S. P., and Xie, J.: Evolutionarily emerged G tracts between the polypyrimidine tract and 3′AG are splicing silencers enriched in genes involved in cancer, BMC genomics, 15, 1143, https://doi.org/10.1186/1471-2164-15-1143, 2014.
Tanaka, N., Aoyama, T., Kimura, S., and Gonzalez, F. J.: Targeting nuclear receptors for the treatment of fatty liver disease, Pharmacol. Therapeut., 179, 142–157, https://doi.org/10.1016/j.pharmthera.2017.05.011, 2017.
Trompier, D., Vejux, A., Zarrouk, A., Gondcaille, C., Geillon, F., Nury, T., Savary, S., and Lizard, G.: Brain peroxisomes, Biochimie, 98, 102–110, 2014.
Varanasi, U., Chu, R., Chu, S., Espinosa, R., LeBeau, M. M., and Reddy, J. K.: Isolation of the human peroxisomal acyl-CoA oxidase gene: organization, promoter analysis, and chromosomal localization, P. Natl. Acad. Sci. USA, 91, 3107–3111, 1994.
Vluggens, A., Andreoletti, P., Viswakarma, N., Jia, Y., Matsumoto, K., Kulik, W., Khan, M., Huang, J. S., Guo, D. S., Yu, S. T., Sarkar, J., Singh, I., Rao, M. S., Wanders, R. J., Reddy, J. K., and Cherkaoui-Malki, M.: Functional significance of the two ACOX1 isoforms and their crosstalks with PPARα and RXRα, Lab. Invest., 90, 696–708, 2010.
Wang, X., Xue, C., Wang, X., Liu, H., Xu, Y., Zhao, R., Jiang, Z., Dodson, M. V., and Chen, J.: Differential display of expressed genes reveals a novel function of SFRS18 in regulation of intramuscular fat deposition, Int. J. Biol. Sci., 5, 28–33, 2009.
Wang, X., Ota, N., Manzanillo, P., Kates, L., Zavala-Solorio, J., Eidenschenk, C., Zhang, J., Lesch, J., Lee, W. P., and Ross, J.: Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes, Nature, 514, 237–241, 2014.
Wu, X.: Capra hircus breed Fuqing ACOX1 splice variant 1 (ACOX1) mRNA, complete cds, alternatively spliced, NBCI GenBank – Nucleotide, Accession number: KX828848, Version KX828848.1, available at: https://www.ncbi.nlm.nih.gov/nuccore/KX828848, last access: 21 September 2017.
Yu, S., Rao, S., and Reddy, J. K.: Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis, Curr. Mol. Med., 3, 561–572, 2003.
Zhang, S. H., Wu, X. F., Pan, C. Y., Lei, C. Z., Dang, R. H., Chen, H., and Lan, X. Y.: Identification of novel isoforms of dairy goat EEF1D and their mRNA expression characterization, Gene, 581, 14–20, 2016.
Zhang, X. Y., Li, M. X., Wu, X. F., Pan, C. Y., Lei, C. Z., Chen, H., and Lan, X. Y.: Novel splice isoforms of dairy goat DBC1 and their diverse mRNA expression profiles, Small Rumin. Res., 130, 15–26, 2015.
Zhang, X. Y., Zhang, S. H., Yang, Q., Lei, C. Z., Chen, H., and Lan, X. Y.: Exploration of dairy goat PITX2 alternative splice events and differential isoform expression, Small Rumin. Res., 144, 140–144, 2016.
Zuo, B., Yang, H., Wang, J., Lei, M. G., and Xiong, Y. Z.: Molecular characterization, sequence variation and association with fat deposition traits of ACOX1 gene in pigs, J. Anim. Feed. Sci., 16, 433–444, 2007.