the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The synergetic effect of selenium or zinc oxide nanoparticles with chromium on mitigating thermal stress for sustainable production and improving antioxidant capacity and inflammatory cytokines of growing rabbits
Ibrahim T. El-Ratel
Khaled H. El-Kholy
Soma M. Elgmmal
Sara Fikry Fouda
Abdel-Khalek E. Abdel-Khalek
Mahmoud A. Hassan
Mahmoud M. Azzam
Mahmoud Alagawany
Antonia Lestingi
This study was conducted to evaluate the impacts of selenium nanoparticles (SeNPs), zinc oxide nanoparticles (ZnONPs), and a combination of SeNPs and chromium (Cr) or ZnONPs and Cr on growth, caecal microbiota, antioxidant capacity in blood and liver tissue, and inflammatory cytokines in heat-stressed rabbits. A total of 100 newly weaned APRI rabbits were randomly divided into five homogeneous groups. A basal diet containing no feed additives (0 g per kg diet) was given to the first group, and the second, third, fourth, and fifth groups were given a diet supplemented with 0.3 mg SeNPs, 20 mg ZnONPs, 0.3 mg SeNPs and 1.5 mg Cr, and 20 mg ZnONPs and 1.5 mg Cr per kg diet, respectively. At 10 and 14 weeks of age, the live body weight (LBW) of rabbits was higher (P < 0.05) in all treatments, while LBW at 10 weeks of age was higher (P < 0.05) in combination groups. All treatments increased daily body weight gain in the age intervals of 6–10 and 6–14 weeks (P<0.05). Daily body weight gain was increased (P < 0.05) in combination groups at the age interval of 6–10 weeks. Feed intake was only increased for rabbits in the ZnONP–Cr group at age intervals of 10–14 weeks. The feed conversion ratio was significantly improved in all treatments at 6–10 and 6–14 weeks of age compared to the control. Haemoglobin was increased (P<0.05) in diets supplemented with ZnONPs and SeNP–Cr or ZnONP–Cr combinations. The platelet count was only increased (P < 0.05) by the ZnONP–Cr combination compared to other groups. Serum total proteins, total antioxidant capacity, superoxide dismutase, glutathione peroxidase, IgA, IgM, nitric oxide, and lysozyme were increased, while serum total cholesterol and triglycerides, alanine transaminase, malondialdehyde, myeloperoxidase, tumour necrosis factor (TNF-α), and interleukin 4 (IL-4) were reduced by all treatments. The total antioxidant capacity in liver tissue was higher, and malondialdehyde was lower in all treatment groups. Albumin was significantly increased, while glucose, creatinine, and urea were significantly decreased in response to ZnONPs and SeNP–Cr or ZnONP–Cr combinations compared with the other groups. Dietary addition of SeNPs–Cr or ZnONPs–Cr significantly reduced interferon-gamma (IFN-γ) concentration. The caecal activity was increased, while the Escherichia coli (E. coli) count decreased considerably in all treatments compared to the control. In conclusion, SeNPs or ZnONPs with chromium as trace elements of growing rabbits can be recommended as an effective intervention to mitigate the negative impacts of heat stress (HS) by enhancing growth performance, promoting metabolic processes, and boosting immunity.
- Article
(455 KB) - Full-text XML
- BibTeX
- EndNote
As a non-ruminant herbivore, rabbits are characterized by high-fibre diets and are considered hindgut digesters. Rabbits are important in Egypt's meat production (Amer et al., 2019). Compared with other livestock, rabbits have a rapid reproductive cycle and growth rate, high meat quality, and high genetic selection potential (Kumar et al., 2018; El-Ratel et al., 2023). Rabbits are susceptible to heat stress (HS) and heat anxiety in hot climates (Madkour et al., 2020). Under HS conditions, changes in oxidative stress, acid–base imbalance, and immunosuppression lead to a reduction in growth performance, including weight gain, feed consumption, feed utilization, and viability of rabbits (Amer et al., 2019; Abdel-Wareth et al., 2022). Also, HS causes oxidative DNA, protein, and lipid damage, leading to an increase in the reactive oxygen species (ROS) generation and a decrease in the body's antioxidant defence system's ability to remove toxins (Prasad and Bao, 2019).
To eliminate the harmful impacts of HS, different feeding strategies by dietary supplementation with phytogenics, amino acids, enzymes, trace minerals, vitamins, prebiotics, or probiotics have been used to enhance animal growth performance (Alagawany et al., 2021a; Abdelnour et al., 2022; El-Ratel et al., 2020). Relating to this, minerals have an important role as nutraceuticals in animal production for their requirement to cover the optimal response of animals to physiological and metabolic actions by increasing the enzymatic and hormonal activities and regulating the acid–base balance and osmotic homeostasis of animals (Weyh et al., 2022; El-Gindy et al., 2023; Mohamed et al., 2023).
Chromium (Cr) is a micronutritional element for metabolizing carbohydrates, lipids, proteins, nucleic acids, and physiological processes (El-Kholy et al., 2017; Mohamed et al., 2023). Cr is a component of chromodulin and an oligopeptide low-molecular-weight Cr-binding substance, which plays a central role in the mechanism of insulin signalling auto-amplification (Amer et al., 2019), leading to increased glucose transport and decreased HS via a reduced level of stress proteins (Piray and Foroutanifar, 2021).
Zinc (Zn) is one of the most crucial and nutritional trace minerals and is included in nucleic acid biosynthesis and cellular division processes (Alagawany et al., 2021b). Also, it has a vital role in physiological functions in the body and has an ability as an antioxidant by scavenging the production of free radicals (Martemucci et al., 2022). It can be used as a dietary supplement to promote bone development, growth, enzyme structure, and immunity in poultry (Madkour et al., 2022; Abdel-Wareth et al., 2022). Moreover, it is pivotal in the metabolism of proteins, fats, and carbohydrates (Kechrid and Bouzerna, 2004) and in gut health, consequently increasing feed utilization (Surai et al., 2017).
Selenium (Se), as an essential trace element, is vital for animal rearing in HS conditions. Se is important for body metabolism, with enzymatic antioxidant properties such as glutathione peroxidase (GPx) to prevent lipid peroxides and ROS from causing cellular damage (Amer et al., 2019). Dietary Se in an appropriate amount is crucial for physiological and biological functions, including productive and reproductive efficiency, immune response, antioxidative action, stress protection, and metabolism of hormones in animals (Qazi et al., 2019; Abdelnour et al., 2022; Liang et al., 2022; El-Ratel et al., 2023). Organic Se has lower toxicity and higher efficacy than inorganic Se in animals under HS conditions (Kim and Kil, 2020).
Nanotechnology is applied in feeding animals, and the most basic approaches in this area are nanoformulations with 1–100 nm dimensions. Nanoformulations have increased bioavailability, specific surface area, surface centre activity, catalytic and adsorptive capacity, and low toxicity (El-Ratel et al., 2023). They are supplemented with diets at low levels more efficiently than their native forms (Hassan et al., 2017), so nanoformulations can be easily assimilated into the digestive tract (Konkol and Ojnarowski, 2018).
Dietary supplementation with a combination of Zn, Se, and Cr was reported to enhance broiler growth performance (Ghasemi et al., 2020). Addition of Zn–Cr in diets up to 3160 mg kg−1 can increase productive efficiency and the physiological status of chicks (Ognik et al., 2020; Zaghari et al., 2023). A dietary combination of Zn–Cr and organic Se reduced the impaired effects of HS on broilers via increased metabolic processes and improved growth efficiency (Mohamed et al., 2023).
In the current study, we hypothesized that nanoparticles of trace elements alone or a combination can mitigate the negative impacts of HS conditions on productive performance parameters, health status markers, and immune response of growing rabbits. Therefore, this study aimed to investigate the modulatory effects of the dietary inclusion of Se or Zn in nanoparticle forms, or a combination of Se nanoparticles and Cr (native form) or Zn nanoparticles and Cr (native form), on the growth performance, caecal activity, haemato-biochemical parameters, antioxidant capacity in blood and liver tissue, immunoglobulins, inflammatory cytokines, and carcass traits of growing rabbits reared under HS conditions.
The Animal, Poultry, and Fish Production Department, Faculty of Agriculture, Damietta University, Egypt, supervised the experimental work for this study at a commercial rabbit farm in Mansoura, Dakahlia Governorate. The European Community norms (EU Directive 2010/63/EU) for the scientific care and use of animals guided all operations.
2.1 Animals
In this work, the experimental animals included 100 APRI rabbits (Egyptian line, Red Baladi × V-line species, selected for litter weight according to Abou Khadiga et al., 2010). They were weaned at 42 d of age and had an average body weight of 694.72±3.324 g. All weaned rabbits underwent a 7 d adaptation period before the start of the trial.
The animals were housed individually in galvanized wire battery cages ( cm) with feeders and automatic drinking nipples. During the experiment, rabbit cages were placed in an open facility with natural ventilation (windows and ceiling fans) and a photoperiod of 16 h of light and 8 h of darkness. All rabbits were subjected to similar management, hygiene, and environmental settings throughout the experiment.
2.2 Experimental design
The rabbits were randomly separated into five groups. A basal diet containing no feed additives (0 g per kg diet) was given to the first group, and the second, third, fourth, and fifth groups were given a diet supplemented with 0.3 mg selenium nanoparticles (SeNPs), 20 mg zinc oxide nanoparticles (ZnONPs), 0.3 mg SeNPs and 1.5 mg Cr (SeNPs–Cr), and 20 mg ZnONPs and 1.5 mg Cr (ZnONPs–Cr) per kg diet, respectively. The experiment lasted 8 weeks during July and August. The basal diet was a full pelleted meal free of antibiotics, commercially used to feed growing rabbits. The ingredients and chemical analysis of the basal diet are reported in Table 1.
Table 1Ingredients and chemical analysis of the basal diet fed to the growing rabbits.
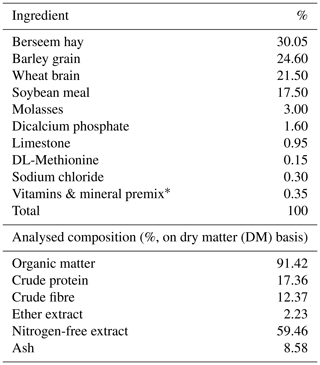
* Each 1 kg contains vitamin A (15 000 IU), vitamin E (100 mg), vitamin B1 (10 mg), vitamin K3 (21 mg), vitamin B2 (40 mg), vitamin B6 (15 mg), vitamin B12 (0.1 mg), pantothenic acid (100 mg), niacin (200 mg), biotin (0.5 mg), folic acid (10 mg), and choline chloride (5000 mg). Each 1 kg contains manganese (800 mg), zinc (600 mg), iron (300 mg), copper (40 mg), iodine (500 mg), selenium (100 mg), and cobalt (100 mg).
2.3 Climate conditions
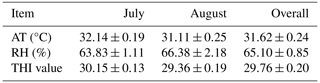
The ambient temperature (AT) and relative humidity (RH) were measured daily at 14:00 PM. An automatic thermohygrometer was used (OF 14:140, H 10 %–99 %; TFA Dostmann GmbH & Co. KG, Wertheim, Germany). During the experiment, temperature–humidity index (THI) values were determined using the formula by Marai et al. (2022): THI = Tdb − [(0.31−0.31(RH)] × [(Tdb − 14.4)], where Tdb is the dry bulb temperature (°C). THI readings range from < 27.8 (lack of HS) to >30.0 (extremely severe HS). The moderate HS is 27.8–28.9.
2.4 Growth performance parameters
During the trial, live body weight (LBW) was measured at 6 (initial), 10, and 14 weeks (final) of age, and the average daily weight growth was determined at age intervals of 6–10, 10–14, and 6–14 weeks. Furthermore, feed intake was recorded separately per day, and the average feed intake over the same intervals was calculated by weighing the residuals of daily given feed. The feed conversion ratio (FCR; g feed / g gain) was also computed for the same age intervals.
2.5 Blood samples
At the end of the trial (14 weeks old), blood samples were carefully collected from five rabbits in each group. After topical anesthesia with 4 % xylocaine, samples were obtained from the marginal ear vein and placed in two test tubes, one with heparin (as an anticoagulant) and the other without.
Whole blood samples were collected in heparinized tubes and used to assess some haematological parameters such as haemoglobin (Hb), the count of red (RBCs) and white (WBCs) blood cells, platelet count, and packed cell volume (PCV) using a blood haematology analyser. The non-heparinized tubes were kept at room temperature for 2 h to clot. Blood samples were centrifuged (T32c; Janetzki, Wallhausen, Germany) at 700× g for 15 min to isolate blood serum in 1.5 mL Eppendorf tubes. The serum was then refrigerated at −20 °C for further biochemical analysis.
Colorimetric analysis was used to assess the concentrations of total protein (TP), albumin (AL), glucose, total cholesterol (TC), triglycerides (TGs), urea, and creatinine in the serum. Commercial chemical kits (BioSystems S.A., Barcelona, Spain) were used for this procedure. However, the globulin (GL) concentration was computed by subtracting the AL values from the corresponding TP values. The activities of aspartate (AST) and alanine (ALT) transaminase in blood serum were determined using commercial kits (BioSystems S.A., Barcelona, Spain). Serum redox status, immunoglobulins, and inflammatory cytokine indicators were determined following the manufacturer directives.
Blood serum samples were tested for total antioxidant capacity (TAC), glutathione (GSH), superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) using commercial kits (Diagnostic, Egypt). In addition, serum myeloperoxidase activity was measured using commercial kits (MBS724170, MyBioSource, San Diego, CA, USA).
ELISA kits measured immunoglobulin concentrations in blood serum, specifically immunoglobulins (IgG, IgM, and IgA). Blood serum levels of interferon-gamma (IFN-γ) (MBS2601171), tumour necrosis factor (TNF-α (MBS7612133), and interleukin 4 (IL-4) (MBS733925) were measured using commercial sandwich ELISA kits (MyBioSource, San Diego, USA). Nitric oxide (NO) and lysosome activity were also measured in blood serum.
2.5.1 Total antioxidant capacity and malondialdehyde levels in liver tissue
After scarification, liver tissue samples were collected from five rabbits per group, homogenized with 10 % w/v potassium phosphate buffer (pH 7.4) solution, and centrifuged at 700× g for 20 min. TAC and MDA levels in supernatants were measured using commercial chemical kits (Diagnostic and Research Reagents, Dokki, Giza, Egypt) and a spectrophotometer (Shimadzu, Japan).
2.6 Carcass characteristics
Five rabbits were randomly chosen from each group after the growth period had ended (14 weeks old). Rabbits were fasted for 12 h and weighed before killing by a trained person. After slaughter and thorough bleeding, the viscera, tail, and pelt were removed, followed by the carcass, head, liver, heart, lung, spleen, and kidneys, which were weighed and calculated as a percentage of pre-slaughter weight. The dressing percentage was obtained by dividing the net carcass weight (hot-dressed) by the pre-slaughter weight times 100.
2.7 Caecal characteristics
After slaughter, caecal contents samples were collected and filtered using an OP-110. The concentration of ammonium nitrogen (NH3-N) and volatile fatty acids (VFAs) was measured. The total microbial and Escherichia coli (E. coli) counts in the caecal content were measured. The pH of the caecal contents was tested using a Radelkis pH meter (Hungary).
2.8 Statistical analysis
The Levene and Shapiro–Wilk tests were used to assess normality and variance homogeneity. The data were statistically evaluated using one-way ANOVA (PROC ANOVA; SAS, 2012 version 8, Cary, NC, USA) to determine the influence of treatment on the various parameters studied. Duncan's multiple range test was used to compare means with a significance threshold of P < 0.05. The results were presented as means ± standard error of the mean (SEM).
3.1 Climate conditions
During the experiment, the rabbits experienced heat stress, averaging 31.62±0.24°C, 65.10 % RH ± 0.85 % RH, and 29.76±0.20 THI (Table 2).
3.2 Growth performance
Table 3 shows the effects of dietary supplementation with SeNPs, ZnONPs, and a combination of SeNPs–Cr or ZnONPs–Cr on the growth performance of heat-stressed rabbits. At 10 and 14 weeks of age, rabbits had greater LBW (P<0.05) in all treatments compared to the control group. At 10 weeks of age, the combination group had higher LBW (P < 0.05) than the SeNP or ZnONP groups. However, the variations in LBW across treatment groups were not significant. All treatments significantly increased average daily gain (P < 0.05) at 6–10 and 6–14 weeks of age compared to the control group. At 6–10 weeks of age, combination groups showed a better average daily increase (P<0.05) compared to SeNP or ZnONP groups. However, the treatment groups did not affect the average daily increase between 10 and 14 weeks of age. At the age interval of 10–14 weeks, only rabbits in the ZnONP–Cr group increased their feed consumption compared to the control group, although this did not differ substantially from the other treatment groups. However, there was no significant effect of treatment on rabbit feed consumption at 6–10 and 6–14 weeks. The feed conversion ratio improved considerably in all treatments compared to the control group at 6–10 and 6–14 weeks of age but not at 10–14 weeks.
Table 3Effects of dietary different sources of trace mineral supplementation on growth performance and feed utilization of growing rabbits exposed to heat stress conditions.
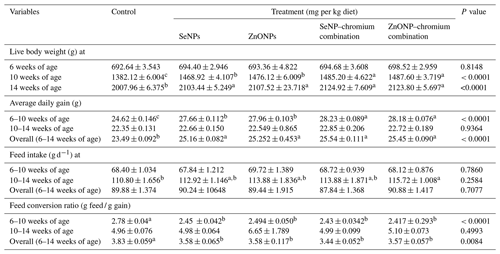
Means (n=20) not sharing a common superscript in a row are significantly different (P<0.05). SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1); SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet); ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet).
Table 4Effects of dietary different sources of trace mineral supplementation on carcass traits of growing rabbits exposed to heat stress conditions.
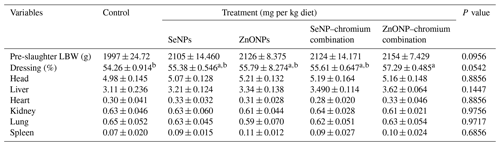
a,b Means not sharing a common superscript in a row are significantly different (P<0.05). SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1), SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet), ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet).
3.3 Carcass criteria
The effect of treatments on carcass features, such as percentage weight of head, liver, heart, kidney, lung, and spleen, was not significant (Table 4). The dressing rate was higher (P < 0.05) in rabbits fed a diet enriched with the ZnONP–Cr combination compared to the control group, but it did not differ substantially from other treatment groups.
3.4 Haemo-biochemical parameters
Table 4 shows how different sources of trace minerals affect haematological and biochemical markers. Diets supplemented with ZnONPs and combined with SeNPs–Cr or ZnONPs–Cr resulted in considerably higher Hb concentrations (P < 0.05). Platelet count increased considerably (P < 0.05) with the ZnONP–Cr combination compared to the SeNP and control groups. Treatments had no significant effect on RBC and WBC counts, as well as the PCV (%). In terms of blood biochemistry, adding SeNPs, ZnONPs, and SeNP–Cr or ZnONP–Cr combinations to growing rabbit diets significantly increased TP concentrations, while it decreased total cholesterol and triglyceride concentrations and ALT activity in serum compared to the control group. In response to ZnONPs and SeNP–Cr or ZnONP–Cr combinations, albumin concentrations increased, while glucose, creatinine, and urea concentrations decreased (P < 0.05) compared to the other groups. Dietary supplements did not significantly affect globulin, the ratio, or AST activity.
Table 5Effect of dietary different sources of trace mineral supplementation on haemato-biochemical parameters of growing rabbits exposed to heat stress conditions.
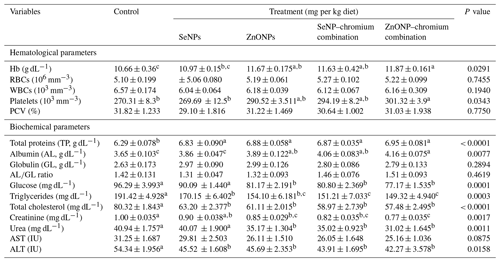
Means (n=5) not sharing a common superscript in a row are significantly different (P < 0.05). AST, aspartate transaminase; ALT, alanine transaminase. SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1), SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet), ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet).
Table 6Effect of dietary different sources of trace mineral supplementation on antioxidant capacity in blood and liver tissue of growing rabbits exposed to heat stress conditions.
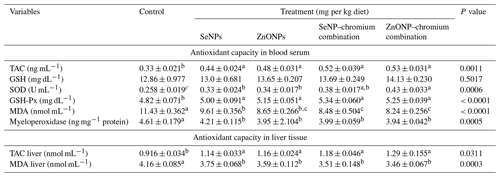
Means (n=5) not sharing a common superscript in a row are significantly different (P < 0.05). SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1), SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet), ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet). TAC, total antioxidant capacity; GSH, glutathione; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde.
3.5 Antioxidant capacity in blood serum and liver tissue
The results in Table 6 reveal that nutritional treatment substantially affects the redox state in the serum and liver tissue of developing rabbits under heat stress. When compared to the control group, all treatments caused raised serum TAC, SOD, and GPx activity and decreased serum MDA and myeloperoxidase activity. TAC levels were significantly higher, while MDA levels were significantly lower in the liver tissue of all treatment groups compared to the control.
3.6 Immunity and inflammatory cytokines
Table 7 shows how different sources of trace minerals affect immunoglobulins and inflammatory cytokines. Dietary supplementation with all supplements significantly raises IgA and IgM concentrations compared to the control (P<0.05). However, supplementation had no significant effect on IgA concentrations. Dietary supplements significantly (P<0.05) enhanced nitric oxide and lysozyme while decreasing TNF-α and IL-4 levels compared to the control group. However, only the dietary addition of SeNP–Cr or ZnONP–Cr combinations significantly lowered IFN-γ concentration relative to the control group but did not differ substantially from the SeNP and ZnONP groups.
Table 7Effect of dietary different sources of trace mineral supplementation on immunoglobulins and inflammatory cytokines of growing rabbits exposed to heat stress conditions.
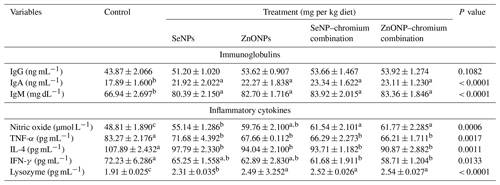
Means (n=5) not sharing a common superscript in a row are significantly different (P < 0.05). SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1), SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet), ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet). IgG, immunoglobulin G; IgM: immunoglobulin M; TNF-α: tumour necrosis factor; IL-4: interleukin 4; IFN-γ, interferon-gamma.
3.7 Caecal characteristics
Table 8 shows that the amounts of ammonia-N and VFAs in the caecal contents of growing rabbits were increased by supplementation of feed additives. At the same time, the E. coli count was reduced in all treatments compared to the control group. Feed additives did not significantly affect the caecal length or pH value of caecal contents.
Table 8Effect of dietary different sources of trace mineral supplementation on caecal activity and E. coli count of growing rabbits exposed to heat stress conditions.
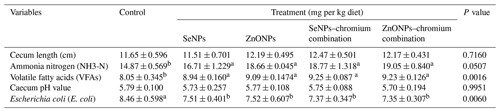
a,b Means (n=5) not sharing a common superscript in a row are significantly different (P<0.05). SeNPs, selenium nanoparticles (0.3 mg per kg diet); ZnONPs, zinc nanoparticles (20 mg kg−1), SeNP–chromium combination (0.3 mg SeNPs per kg diet + 1.5 mg chromium per kg diet), ZnONP–chromium combination (20 mg ZnONPs per kg diet + 1.5 mg chromium per kg diet).
Rabbits are vulnerable to HS and have few sweat glands, making it difficult to cool their bodies (Marai et al., 2002). Hot climates impact rabbit productivity and physiological states (Jaen-Tellez et al., 2021). Our study found that growing rabbits experienced HS during the experimental period, as shown by the computed THI value of 29.76±0.20. Dietary trace mineral supplementation is required to ameliorate the negative effects of HS on poultry (Abdel-Wareth et al., 2022; El-Ratel et al., 2023; Mohamed et al., 2023). In this study, the hypothesis is that supplementation with different trace minerals, such as SeNPs, ZnONPs, SeNPs–Cr, or ZnONPs–Cr, may have a positive influence on the growth performance, feed utilization, and health status of growing rabbits, hence mitigating the harmful effects of HS. These trace minerals are excellent feed additions with various potential medicinal effects, including antioxidant, antibacterial, antiviral, anti-inflammatory, and hepatoprotective qualities for rabbits growing under HS conditions (Abdel-Wareth et al., 2022; El-Ratel et al., 2023; Mohamed et al., 2023). In the current study, dietary supplementation of SeNPs, ZnONPs, and SeNPs–Cr or ZnONPs–Cr combinations improved rabbit growth performance parameters at 6–10 weeks of age as compared to the control group, in terms of higher LBW at 10 weeks of age, higher daily gain, and no significant changes in feed intake at the age interval of 6–10 weeks. Increased LBW of rabbits at 10 weeks of age, minor changes in daily gain at 10–14 weeks, and increased feed intake in treatment groups resulted in no significant differences in feed conversion ratio between treatment groups and the control group under HS circumstances. However, adding the ZnONP–Cr combination increased the dressing rate (P < 0.05) in rabbits compared to the control group but did not differ substantially from other treatment groups. In this regard, Mohamed et al. (2023) suggest that trace elements like Zn–Cr and organic Se may significantly reduce the effects of HS on broilers by improving growth performance and carcass features. In addition, Jain et al. (2020) found that Zn–Cr and Se increased the feed conversion ratio in broilers. In growing rabbits, dietary supplementation with ZnONPs can reduce the deleterious effects of HS on productivity (Kamel et al., 2020; Abdel-Wareth et al., 2022). Dietary ZnONPs have been demonstrated to help reduce the deleterious effects of HS in poultry (Ramiah et al., 2019; Shokraneh et al., 2020). Many authors have demonstrated the promising favourable benefits of ZnONPs for animal performance (Abdelnour et al., 2021; Cui et al., 2021). Se nanoparticles, particularly when manufactured biologically, increased the growth performance and carcass features of growing rabbits during thermal stress (Sheiha et al., 2020), and feeding Se nanoparticles improves rabbit productivity during HS (El-Badry et al., 2019). Dietary supplementation with SeNPs may improve the growth performance of broilers raised under HS conditions (El-Deep et al., 2016). The improvement in weight gain may be attributed to the fact that trace minerals can increase intestinal trace element absorption by reducing interference from substances that form insoluble complexes with ionic trace elements, thereby improving their bioavailability and body weight development (Jain et al., 2020). Furthermore, dietary trace element supplementation may increase feed utilization and nutrient absorption compared to the control diet in broilers with poor growth performance under HS, which spent more energy to adapt to the stressor than to grow (Alwaleed et al., 2021).
Enhanced growth performance in this study may be connected to improved feed consumption and nutrient digestibility. In this regard, trace element supplementation increases nutritional digestibility, resulting in a higher FCR (Sahin et al., 2009). In the current study, SeNPs, ZnONPs, and a combination of SeNPs–Cr or ZnONPs–Cr improved growth performance due to the trace elements' critical role in the molecular structure of enzymes and proteins, serving as co-enzymes and activators that are primarily involved in physiological functions (Nguyen et al., 2021). Zn is widely recognized for increasing the activity of the insulin-like growth factor and growth hormone genes, as well as for controlling hunger and improving nutrient performance and digestibility (Ibrahim et al., 2017). Cr also plays a role in the metabolism of glucose, lipids, proteins, and nucleic acids, in addition to its growth-promoting actions, and it enhances the biological function of insulin-sensitive cell receptors by boosting their binding activity (Mohamed et al., 2023).
In this study, heat-stressed rabbit diets fortified with ZnONPs and a combination of SeNPs–Cr or ZnONPs–Cr increased Hb concentration and platelet count significantly (P < 0.05) compared to other groups. This study improved heat-stressed broiler chicks' haematological parameters by dietary addition of Se yeast alone or in combination with Zn–Cr (Mohamed et al., 2023). The significant variations in Se, Zn, or Cr related to haematological parameters could be attributed to Se's positive influence on increasing Hb concentration (Mohamed et al., 2023) and Zn's participation in erythropoiesis, which leads to an increase in Hb when broilers are fed zinc-supplemented diets. Zn is also a catalytic agent for α-aminolevulinic acid dehydrogenase, an enzyme implicated in heme formation (Aksu et al., 2010). The increase in Hb content in treatment groups could be attributed to Zn–Cr and Se–Cr's antioxidant capabilities and their ability to enhance enzyme synthesis, stability, and activity in the body (Mohamed et al., 2023). In terms of blood biochemicals, adding SeNPs, ZnONPs, and SeNP–Cr or ZnONP–Cr combinations to growing rabbit diets significantly increased total protein concentration, decreased total cholesterol and triglyceride concentrations, and decreased serum ALT activity when compared to the control group. ZnONPs and SeNP–Cr or ZnONP–Cr combinations significantly raised albumin concentrations, while they decreased glucose, creatinine, and urea concentrations (P<0.05) compared to other groups. Reducing ALT activity implies improved liver function, mitigating the negative effects of a heated environment (Kew, 2002; Sheiha et al., 2020). These improvements in rabbit serum biochemistry may be related to zinc being a component of Zn metalloenzymes, which maintain protein structural integrity (Chrastinová et al., 2015). Se can reduce the formation of thiobarbituric-acid-reactive substances while increasing the activity of glutathione S-transferase and total sulfhydryl groups, which are necessary components of the enzyme glutathione peroxidase, which in turn prevents lipid and protein oxidation (El-Demerdash, 2004). This impact could be attributable to all supplements reducing cholesterol absorption and synthesis in the poultry gut (Abd El-Hack et al., 2019). Se nanoparticles can improve the lipid profile, possibly due to its protective properties against HS.
Furthermore, selenium-coated nanostructures are helpful in the treatment of fatty liver disease by increasing oral bioavailability and improving the hypoglycemic effects of phytogenesis (Hosnedlova et al., 2018). Chromium is an essential mineral that is an integral component of chromodulin and is required for insulin activity (Vincent, 2000). The Cr can enhance glucose transporter for transfer from the cytoplasm to the cell membrane by activating insulin receptors and facilitating glucose entrance into cells (Vincent 2015). Furthermore, Cr is a key component of the glucose tolerance factor, which influences glucose metabolism, lipids, proteins, and nucleic acids by enhancing insulin activity (Hayirli, 2005). Combining Zn–Cr and organic Se increased broiler chicks' blood biochemical and metabolic responses, including high total protein and LDH (lactate dehydrogenase) and low glucose, triglycerides, LDL (low-density lipoprotein), creatinine, and uric acid (Mohamed et al., 2023). ZnONP supplementation effectively (P < 0.05) reduced blood cholesterol, creatinine, and urea concentrations, as well as AST and ALT activity, in developing rabbits under HS circumstances (Abdel-Wareth et al., 2022). Furthermore, SeNPs play an important role in enhancing rabbit blood metabolites under HS circumstances (Bashar et al., 2022) and renal function by lowering uric acid and creatinine concentrations (Sheiha et al., 2020). In line with our findings, Cr-propionate supplementation reduced serum hyperglycemia in broiler chicks compared to the control group but did not affect AST and ALT activity (Arif et al., 2019).
In rabbits exposed to HS, serum antioxidant enzyme activity was lowered, and MDA levels increased, resulting in organ damage and decreased productivity (El-Ratel et al., 2020, 2023). In the current study, all treatments raised serum TAC content, SOD, and GPx activities while decreasing serum MDA levels and myeloperoxidase activity as compared to the control group. TAC levels were significantly greater in liver tissue, but MDA levels were significantly lower in all treatment groups compared to the control. HS is widely known to induce ROS formation, linked to apoptosis and other disorders caused by lipid peroxidation damage to DNA, proteins, and cell phospholipid membranes (Abdel-Wareth et al., 2022). Trace elements may reduce the negative effects of HS on animal health by reducing free radicals and inhibiting lipid peroxidation (Ai et al., 2009; El-Ratel et al., 2023). Supplementation with Zn–Cr and/or organic Se significantly increased serum antioxidant activity by boosting TAC content and decreasing MDA levels compared to the non-supplemented group of broiler chicks (Mohamed et al., 2023). Also, supplementing broilers' diets with organic forms of 0.3 mg Se, 2 mg Cr, and 40 mg Zn kg−1 (individually or in combination) significantly increased their antioxidant responses to SOD and MDA during the hot summer season (Rao et al., 2016).
Increasing the TAC content and activity of SOD and GPx, as well as the reduction in MDA levels in serum of supplemented rabbits under HS circumstances, demonstrated significant benefits of SeNP–Cr or ZnONP–Cr combinations as dietary supplements in limiting the detrimental effects of HS. Zn is a powerful indirect antioxidant that prevents free radical formation and slows oxidative processes (Vlaicu et al., 2022). Furthermore, Zn supplementation reduces free radicals since it is a component of SOD and GSH, which play an important role in the formation of metallothionein and act as free radical scavengers (Lee, 2018; Liang et al., 2022). Accordingly, Zn is a key element that must be included in poultry diets under HS (Liang et al., 2022). These findings suggested that Zn is an anti-heat stress agent and is primarily found in the forms of Zn monocarbonate, Zn sulfate monohydrate, Zn pyridine acid, bacitracin Zn, granular coated bacitracin Zn, and amino acid Zn (Liang et al., 2022).
On the other hand, dietary Se nanoparticle supplementation improved antioxidant activities in rabbits by enhancing GSH activity and decreasing MDA levels compared to the heat-stressed group (Sheiha et al., 2020). Se is a crucial component of various Se proteins, including GPx and thioredoxin reductase (Zhou et al., 2013); hence, the improved antioxidant capability may be attributable to inducible Se-dependent antioxidant enzymes. In this regard, the GPx is a prominent phase II detoxification enzyme that may reduce the degree of lipid peroxidation, and dietary inclusion of a sufficient quantity of Se was discovered to be critical in scavenging oxidation and boosting immunity in rabbits (Liang et al., 2022). Similarly, trace minerals offer potential medicinal activity against oxidation, bacteria, viruses, and inflammation, in addition to hepatoprotective qualities (Abdel-Wareth et al., 2023; El-Ratel et al., 2023; Mohamed et al., 2023).
Under HS circumstances, a significant negative effect on the immunological response in rabbits was observed via the hypothalamic–pituitary–adrenal axis (Elazab et al., 2022) to elicit glucocorticoid functions as an anti-immunity element (Liang et al., 2022). Increasing glucocorticoid concentrations changes both the cellular and humoral immune systems. As a result, HS has a negative impact on rabbit production, including decreased immunity and susceptibility to infections (Marai et al., 2002). In our investigation, dietary supplementation with SeNPs, ZnONPs, and a combination of SeNPs–Cr or ZnONPs–Cr boosted the cellular and humoral immune systems of heat-stressed developing rabbits, as evidenced by a significant rise in serum IgA, IgM, and nitric oxide concentrations, as well as lysozyme activity. The observed increase in lysozyme activity could be attributed to its enzymatic degenerative ability to remove infections (Hashem et al., 2021).
Furthermore, the beneficial effects of these trace minerals on immunity can be related to their diverse biological capabilities, which include antioxidant, antibacterial, antiviral, anti-inflammatory, and hepatoprotective qualities (Abdel-Wareth et al., 2022; El-Ratel et al., 2023; Mohamed et al., 2023). Along with their favourable effects on the immune response of growing rabbits, trace minerals can decrease the de novo production of inflammatory cytokines and, hence, inflammation reactions. HS increases the apical content of pro-inflammatory cytokines.
Abd El-Hack et al. (2020) found that IFN-γ and IL-4 increased intestinal permeability to infections. Our study found that dietary SeNPs, ZnONPs, and SeNPs–Cr or ZnONPs–Cr combinations significantly reduced pro-inflammatory cytokines TNF-α and IL-4 in heat-stressed developing rabbits. The dietary addition of SeNP–Cr or ZnONP–Cr combinations dramatically lowered IFN-γ levels relative to the control group but did not differ significantly from the SeNP and ZnONP groups. These findings suggested that these minerals had potential anti-inflammatory properties, indicating their efficacy in improving the health of animals suffering from HS. SeNPs (25 or 50 mg per kg diet) improved inflammatory cytokines in developing rabbits exposed to temperature stress (Sheiha et al., 2020). Improving animal tolerance to environmental stressors is an important step toward eradicating or avoiding viral illnesses by encouraging immune enhancers and stimulants (Alagawany et al., 2017).
As a result, we investigated the influence of various supplements on the caecal activity of growing rabbits and discovered that ammonia-N and VFA concentrations in caecal contents increased significantly, with a drop in E. coli count by all dietary supplements compared to the control diet. The antimicrobial action of trace elements in decreasing E. coli count in caecal contents may be related to the presence of bioactive substances that hinder microbial growth and disrupt specific metabolic processes. These findings showed that employing SeNPs, ZnONPs, or a combination of SeNPs–Cr and ZnONPs–Cr as feed additives was safe for microbial fermentation in rabbit cecum.
Overall, the results of this study revealed that SeNPs, ZnONPs, and the combination of SeNPs–Cr or ZnONPs–Cr in heat-stressed young rabbits may increase growth rate, carcass characteristics, haematological parameters, biochemical indicators, antioxidant levels, immunology, and inflammatory cytokines. This could be owing to the enhanced bioavailability of certain trace elements after being fabricated in nanoform. SeNP–Cr or ZnONP–Cr combinations are active components that play essential roles as thermoregulatory agents and regulate several physiological activities in rabbits during their growth period. More research is needed to determine how these trace elements affect the reproductive performance of male and female rabbits.
The data presented in this study are available on request from the corresponding author.
ITE-R, KHE-K, SME, SFF, AKEA-K, and MAH designed the study and supervised the experiments. Data were analysed by ITE-R, KHE-K, SME, SFF, AKEA-K, and MAH. The manuscript was prepared and edited by ITE-R, KHE-K, MMA, MA, and AL. All authors contributed to the article and approved the submitted version.
The contact author has declared that none of the authors has any competing interests.
The authors confirm that the ethical policies of Archives Animal Breeding, as noted on the journal's author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received from the institutional committee of Damietta University. Moreover, the authors confirm that they have followed EU standards for the protection of animals used for scientific purposes and feed legislation.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
This work was supported by the Researchers Supporting Project (RSPD2024R731), King Saud University (Riyadh, Saudi Arabia).
This research has been supported by the King Saud University (grant no. RSPD2024R731).
This paper was edited by Manfred Mielenz and reviewed by Olatunji Abubakar Jimoh and one anonymous referee.
Abd El-Hack, M. E., Alagawany, M., and Abdelnour, S.: Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter, J. Anim. Physiol. Anim. Nutr.(Berl)., 103, 509–517, https://doi.org/10.1111/jpn.13045, 2019.
Abd El-Hack, M. E., Abdelnour, S. A., Taha, A. E., Khafaga, A. F., Arif, M., Ayasan, T., Swelum, A. A., Abukhalil, M. H., Alkahtani, S., and Aleya, L.: Herbs as thermoregulatory agents in poultry: An overview, Sci. Total. Environ., 703, 134399, https://doi.org/10.1016/j.scitotenv.2019.134399, 2020.
Abdelnour, S. A., Alagawany, M., Hashem, N. M., Farag, M. R., Alghamdi, E. S., Hassan, F. U., Bilal, R. M., Elnesr, S. S., Dawood, M. A. O., and Nagadi, S. A.: Nanominerals: fabrication methods, benefits and hazards, and their applications in ruminants with special reference to selenium and zinc nanoparticles, Animals., 11, 1916, https://doi.org/10.3390/ani11071916, 2021.
Abdelnour, S. A., El-Ratel, I. T., Peris, S. I., El-Raghi, A. A., and Fouda S. F.: Effects of dietary thyme essential oil on blood haematobiochemical, redox status, immunological and reproductive variables of rabbit does exposed to high environmental temperature, Ital. J. Anim. Sci. 21, 51–61, https://doi.org/10.1080/1828051X.2021.2006807, 2022.
Abdel-Wareth, A. A. A., Amer, S. A., Mobashar, M., and El-Sayed, H. G. M.: Use of zinc oxide nanoparticles in the growing rabbit diets to mitigate hot environmental conditions for sustainable production and improved meat quality, BMC Vet. Res., 18, 354, https://doi.org/10.1186/s12917-022-03451-w, 2022.
Abdel-Wareth, A. A. A., El-Sayed, H. G. M., Abdel-Warith, A.-W. A., Younis, E. M., Hassan, H. A., Afifi, A. S., El-Chaghaby, G. A., Rashad, S., Amer, S. A., and Lohakare, J.: Effects of dietary acacia nilotica fruit, zinc oxide nanoparticles and their combination on productive performance, zinc retention, and blood biochemistry of rabbits, Animals, 13, 3296, https://doi.org/10.3390/ani13203296, 2023.
Abou Khadiga, G., Youssef, Y. M. K., Saleh, K., Nofal, R. Y., and Baselga, M.: Genetic trend in selection for litter weight in two maternal lines of rabbits in Egypt, World Rabbit Sci., 18, 27–32, https://doi.org/10.4995/wrs.2010.18.04, 2010.
Ai, D., Wang, Z., and Zhou, A.: Protective effects of Nano-ZnO on the primary culture mice intestinal epithelial cells in in vitro against oxidative injury. J. Anim. Vet. Adv., 8, 1964–1967, 2009.
Aksu, D. S., Aksu, T., and Ozsoy, B.: The effects of lower supplementation levels of organically complexed minerals (zinc, copper and manganese) versus inorganic forms on hematological and biochemical parameters in broilers, Kafkas Univ. Vet Fak. Derg., 16, 553–559, 2010.
Alagawany, M., Elnesr, S. S., Farag, M. R., Tiwari, R., Yatoo, M. I., Karthik, K., Michalak, I., and Dhama, K.: Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health – A comprehensive review, Vet. Quart., 41, 1–29, 2021a.
Alagawany, M., Qattan, S. Y., Attia, Y. A., El-Saadony, M. T., Elnesr, S. S., Mahmoud, M. A., and Reda, F. M.: Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes, Animals, 11, 3027, doi: 10.3390/ani11113027, 2021b.
Alagawany, M., Farag, M. R., Abd El-Hack, M. E., Dhama, K., and Fowler, J.: Use of acetylsalicylic acid as a feed additive in poultry nutrition, World. Poult. Sci. J., 73, 633–642, 2017.
Alwaleed, E. A., El-Sheekh, M., Abdel-Daim, M. M., and Saber, H.: Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens, Environ. Sci. Pollut. Res., 28, 1801–1811, https://doi.org/10.1007/s11356-023-26610-4, 2021.
Amer, A. Shimaa., Omar, A. E., and Abd El-Hack, M. E.: Effects of selenium- and chromium-enriched diets on growth performance, lipid profile, and mineral concentration in different tissues of growing rabbits, Biol. Trace Elem. Res., 187, 92–99, DOI: https://doi.org/10.1007/s12011-018-1356-4, 2019.
Arif, M., Hussain, I., Mahmood, M. A., Abd El-Hack, M. E., Swelum, A. A., Alagawany, M., and Komany, A.: Effect of varying levels of chromium propionate on growth performance and blood biochemistry of broilers, Animals, 9, 935, https://doi.org/10.3390/ani9110935, 2019.
Bashar, A. M., Abdelnour, S. A., El-Darawany, A. A., and Sheiha Asmaa M.: effect of selenium nanoparticles and/or spirulina platensis on growth, hematobiochemical, antioxidant status, hormonal profile, immunity, and apoptosis of growing rabbits exposed to thermal stress, Egypt. J. Rabbit Sci., 32, 77–103, 2022.
Chrastinová, L., Čobanová, K., Chrenková, M., Poláčiková, M., Formelová, Z., and Lauková, A.: High dietary levels of zinc for young rabbits, Slovak J. Anim. Sci., 48, 57–63, 2015.
Cui, Y., Tian, Z., Lu, H., Deng, D., Liu, Z., Rong, T., Yu, M., and Ma, X.: Zinc oxide nanoparticles improve gut health and reduce faecal zinc excretion in piglets, Livestock Sci., 251, 104610, https://doi.org/10.1016/j.livsci.2021.104610, 2021.
Elazab, M. A., Khalifah, A. M., Elokil, A. A., Elkomy, A. E., Rabie, M. M., Mansour, A. T., and Morshedy, S. A.: Effect of dietary rose-mary and ginger essential oils on the growth performance, feed utilization, meat nutritive value, blood biochemicals, and redox status of growing NZW rabbits, Animals, 12, 375, https://doi.org/10.3390/ani12030375, 2022.
El-Badry, A. S. O., Hassanane, M. M., Mosalm, G. A. G., Ahmed, E. S., and El-Aasar, T. A.: Influence of ingestion of nano-selenium on growth performance, antioxidative and mutagenicity status in somatic cells of new zealand white rabbits, Egypt, J. Rabbit Sci., 29, 1–21, 2019.
El-Deep, M. H., Daichi, I., Ebeid,T. A., and Ohtsuka, A.: Effects of dietary nano-selenium supplementation on growth performance, antioxidative status, and immunity in broiler chickens under thermoneutral and high ambient temperature conditions, J. Poult. Sci., 53, 274–283, https://doi.org/10.2141/jpsa.0150133, 2016.
El-Demerdash, F. M.: Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium, J. Trace Elem. Med. Biol., 18, 113–121, https://doi.org/10.1016/j.jtemb.2004.04.001, 2004.
El-Gindy, Y. M., Zahran, S. M., Ahmed, M. H., Ali, A. M., Mohamed, A. Z., and Morshedy, S. A.: Counteract severe heat stress by including different forms of zinc in the rabbit bucks' diet, Sci. Rep., 13, 12987, https://doi.org/10.1038/s41598-023-39928-3, 2023.
El-Kholy, M. S., El-Hindawy, M. M., Alagawany, M., Abd El-Hack, M. E., and El-Sayed, S. A. A.: Dietary supplementation of chromium can alleviate negative impacts of heat stress on performance, carcass yield, and some blood hematology and chemistry indices of growing Japanese quail, Biol. Trace Elem. Res., 179, 148–157, https://doi.org/10.1007/s12011-017-0936-z, 2017.
El-Ratel, I. T., Tag El-Din, H. T., and Bedier, M. M.: Beneficial effects of curcumin as a native or nanoparticles form on productive efficiency, liver and kidney functions, antioxidative status and immunity of heat-stressed growing rabbits, J. Anim. Physiol. Anim. Nutr., 104, 1778–1787, https://doi.org/10.1111/jpn.13420, 2020.
El-Ratel, I. T., Elbasuny, M. E., El-Nagar, H. A., Abdel-Khalek, A. E., El-Raghi, A. A, El-Basuini, M. F., El-Kholy, Kh. H., and Fouda, S. F.: The synergistic impact of Spirulina and selenium nanoparticles mitigates the adverse effects of heat stress on the physiology of rabbits bucks, PLOS ONE, 18, e0287644, https://doi.org/10.1371/journal.pone.0287644, 2023.
Ghasemi, H. A., Hajkhodadadi, I., Hafizi, M., Taherpour, K., and Nazaran, M. H.: Effect of advanced chelate technology-based trace minerals on growth performance, mineral digestibility, tibia characteristics, and antioxidant status in broiler chickens, Nutr. Metab., 17, 94, https://doi.org/10.1186/s12986-020-00520-5, 2020.
Hashem, N. M., Morsy, A. S., Soltan, Y. A., and Sallam, S. M.: Potential Benefits of Boswellia sacra resin on immunity, metabolic status, udder and uterus health, and milk production in transitioning goats, Agriculture, 11, 900, https://doi.org/10.3390/agriculture11090900, 2021.
Hassan, F., Mahmoud, R., and El-Araby, I.: Growth performance, serum biochemical, economic evaluation and il6 gene expression in growing rabbits fed diets supplemented with zinc nanoparticles, Zagazig Vet. J., 45, 238–249, https://doi.org/10.21608/zvjz.2017.7949, 2017.
Hayirli, A.: Chromium nutrition of livestock species, Nutr. Abstr. Rev., 75, 1–14, 2005.
Hosnedlova, B., Kepinska, M., Skalickova, S., Fernández, C., Ruttkay-Nedecky, B., Peng, Q., Baron, M., Melcova, M., Opatrilova, R., and Zidkova, J.: Nano-selenium and its nanomedicine applications: A critical review, Int. J. Nanomed., 13, 2107–2128, https://doi.org/10.2147/IJN.S157541, 2018.
Ibrahim, D., Ali, H., and El-Mandrawy, S.: Effects of different zinc sources on performance, bio distribution of minerals and expression of genes related to metabolism of broiler chickens, Zagazig Vet. J., 45, 292–304, https://doi.org/10.21608/zvjz.2017.7954, 2017.
Jaen-Tellez, J. A., Sanchez-Guerrero, M. J., Valera, M., and Gonzalez-Redondo, P.: Influence of stress assessed through infrared thermography and environmental parameters on the performance of fattening rabbits, Animals, 11, 1747, https://doi.org/10.3390/ani11061747, 2021.
Jain, A. K., Mishra, A., Caesar, D. D., Shakkarpude, J., Mourya, A., Baghel, R. P. S., and Sharma, R. K.: Can different concentration of chelated and inorganic trace minerals (Zn, Se and Cr) be an effective supplement for better production performance and carcass traits in broilers?, J. Entomol. Zool. Stud., 8, 197–204, 2020.
Kamel, Doaa A., Abdel-Khalek, A. E., and Gabr, Sh. A.: Effect of dietary zinc-oxide or nano-zinc oxide on growth performance, oxidative stress, and immunity of growing rabbits under hot climate conditions, J. Anim. Poult. Prod., 12, 565–571, https://doi.org/10.21608/JAPPMU.2020.161193, 2020.
Kechrid, Z. and Bouzerna, N.: Efect of zinc defciency on zinc and carbo- hydrate metabolism in genetically diabetic (C57BL/Ksj Db+/Db+) and non-diabetic original strain (C57BL/Ksj) mice, Turk. J. Med. Sci., 34, 367–73, 2004.
Kew, M. C.: Serum aminotransferase concentration as evidence of hepatocellular damage, Lancet., 355, 591–592, https://doi.org/10.1016/S0140-6736(99)00219-6, 2000.
Kim, J. H. and Kil, D. Y.: Comparison of toxic effects of dietary organic or inorganic selenium and prediction of selenium intake and tissue selenium concentrations in broiler chickens using feather selenium concentrations, Poult. Sci., 99, 6462–6473, https://doi.org/10.1016/j.psj.2020.08.061, 2020.
Konkol, D. and Ojnarowski, K.: The use of nanominerals in animal nutrition as a way to improve the composition and quality of animal products, J. Chem., 2018, 1–7, https://doi.org/10.1155/2018/5927058, 2018.
Kumar, S. D., Singh, D. A. P., Natarajan, A., and Sivakumar, K.: Carcass characteristics of soviet chinchilla rabbits supplemented with vitamin C, E and selenium during the period of heat stress, Int. J. Curr. Microbiol. Appl. Sci., 7, 1962–1969, https://doi.org/10.20546/ijcmas.2018.708.226, 2018.
Lee, S. R.: Critical role of zinc as either an antioxidant or a prooxidant in cellular systems, Oxid Med Cell Longevity., 20, 9156285, https://doi.org/10.1155/2018/9156285, 2018.
Liang, Z. L., Chen, F., Park, S., Balasubramanian, B., and Liu, W. C.: Impacts of heat stress on rabbit immune function, endocrine, blood biochemical changes, antioxidant capacity and production performance, and the potential mitigation strategies of nutritional intervention, Front. Vet. Sci., 9, 906084, https://doi.org/10.3389/fvets.2022.906084, 2022.
Madkour, M, Aboelenin, M. M., Younis, E., Mohamed, M. A., Hassan, H., Alagawany, M., and Shourrap, M.: Hepatic acute-phase response, antioxidant biomarkers and DNA fragmentation of two rabbit breeds subjected to acute heat stress, Ital. J. Anim. Sci., 19, 1558–1566, 2020.
Madkour, M., Salman, F. M., El-Wardany, I., Abdel-Fattah, S. A., Alagawany, M., Hashem, N. M., Abdelnour, S. A., El-Kholy, M. S., and Dhama, K.: Mitigating the detrimental effects of heat stress in poultry through thermal conditioning and nutritional manipulation, J. Therm. Biol., 103, 103169, 2022.
Marai, I., Habeeb, A., and Gad, A.: Rabbits' productive, reproductive and physiological performance traits as affected by heat stress: A review, Livest. Prod. Sci., 78, 71–90, https://doi.org/10.1016/S0301-6226(02)00091-X, 2022.
Martemucci, G., Costagliola, C., Mariano, M., D'andrea, L., Napolitano, P., and D'Alessandro, A.: Free radical properties, source and targets, antioxidant consumption and health, Oxygen, 2, 48–78, 2002.
Mohamed, A. S. A., Abd El Latif, M. A., Hussein, E. A. M., Toson, E. M. A., Saleh, M., Kokoszynski, D., Elnesr, S. S., Mohany, M., Al-Rejaie, S. S., and Elwan, H.: Efficacy of dietary supplementation with zinc-chromium mixture, organic selenium, or their combinations on growth performance, carcass traits, and blood profiles of broilers under heat stress conditions, Animals, 13, 2539, https://doi.org/10.3390/ani13152539, 2023.
Nguyen, H. T. T., Morgan, N., Roberts, J. R., Wu, S. B., Swick, R. A., and Toghyani M.: Zinc hydroxychloride supplementation improves tibia bone development and intestinal health of broiler chickens, Poult. Sci., 100, 1–9, https://doi.org/10.1016/j.psj.2021.101254, 2021.
Ognik, K., Drażbo, A., Stępniowska, A., Kozłowski, K., Listos, P., and Jankowski, J.: The effect of chromium nanoparticles and chromium picolinate in broiler chicken diet on the performance, redox status and tissue histology, Anim. Feed Sci. Technol., 259, 114326, https://doi.org/10.1016/j.anifeedsci.2019.114326, 2020.
Piray, A. and Foroutanifar, S.: Chromium supplementation on the growth performance, carcass traits, blood constituents, and immune competence of broiler chickens under heat stress: A systematic review and dose–response meta-analysis, Biol. Trace Elem. Res., 200, 2876–2888, https://doi.org/10.1007/s12011-021-02885-x, 2021.
Prasad, A. S. and Bao, B.: Review, molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications, Antioxidants, 8, 164, https://doi.org/10.3390/antiox8060164, 2019.
Qazi, I. H., Angel, C., Yang, H., Zoidis, E., Pan, B., and Wu, Z.: Role of selenium and selenoproteins in male reproductive function: a review of past and present evidences, Antioxidants, 8, 268, https://doi.org/10.3390/antiox8080268, 2019.
Ramiah, S. K., Atta, A. E., Mookiah, S., and Idrus, Z.: Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions, Poult Sci., 98, 3828–3838, https://doi.org/10.3382/ps/pez093, 2019.
Rao, S. V., Prakash, B., Raju, M. V. L. N., Panda, A. K., Kumari, R. K., and Reddy, E.: Effect of supplementing organic forms of zinc, selenium and chromium on performance, antioxidant and immune responses in broiler chicken reared in tropical summer, Biol. Trace Elem. Res., 172, 511–520, https://doi.org/10.1007/s12011-015-0587-x, 2016.
Sahin, K., Sahin, N., Kucuk, O., Hayirli, A., and Prasad, A. S.: Role of dietary zinc in heat-stressed poultry: A review, Poult. Sci., 88, 2176–2183, https://doi.org/10.3382/ps.2008-00560, 2009.
Sheiha, A. M., Abdelnour, S. A., Abd El-Hack, M. E., Khafaga, A. F., Metwally, K. A., Ajarem, J. S., Maodaa, S. N., Allam, A. A., and El-Saadony, M. T.: Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress, Animals (Basel), 10, 430, https://doi.org/10.3390/ani10030430, 2020.
Shokraneh, M., Sadeghi, A. A., Mousavi, S. N., Esmaeilkhanian, S., and Chamani, M.: Effects of in ovo injection of nano-selenium and nano-zinc oxide and high eggshell temperature during late incubation on antioxidant activity, thyroid and glucocorticoid hormones and some blood metabolites in broiler hatchlings, Acta Sci. Anim. Sci., 42, e46029, https://doi.org/10.4025/actascianimsci.v42i1.46029, 2020.
Surai, P. F., Kochish, I. I., and Velichko, O. A.: Nano-Se assimilation and action in poultry and other monogastric animals: is gut microbiota an answer, Nanoscale Res Lett., 12, 1–7, https://doi.org/10.1186/s11671-017-2383-3, 2017.
Vincent, J. B.: Is the pharmacological mode of action of chromium (III) as a second messenger, Biol Trace Elem Res., 166, 7–12. 10.1007/s12011-015-0231-9, 2015.
Vincent, J. B.: The biochemistry of chromium, J. Nutr., 130, 715–718, https://doi.org/10.1093/jn/130.4.715, 2000.
Vlaicu, P. A., Untea, A. E., Turcu, R. P., Saracila, M., Panaite, T. D., and Cornescu, G. M.: Nutritional composition and bioactive compounds of basil, thyme and sage plant additives and their functionality on broiler thigh meat quality, Foods, 11, 1105, https://doi.org/10.3390/foods11081105, 2022.
Weyh, C., Krüger, K., Peeling, P., and Castell, L.: The role of minerals in the optimal functioning of the immune system, Nutrients, 14, 644, https://doi.org/10.3390/nu14030644, 2022.
Zaghari, M., Pouraghaali, S., Zhandi, M., and Abbasi, M.: Effect of monovalent copper oxide and potentiated zinc oxide on growth performance and gut morphology of broiler chickens challenged with coccidiosis, Biol. Trace Elem. Res., 201, 2524–2535, https://doi.org/10.1007/s12011-022-03339-8, 2023.
Zhou, J., Huang, K., and Lei, X. G.: Selenium and diabetes – Evidence from animal studies, Free Radic Biol Med., 65, 1548–1556, https://doi.org/10.1016/j.freeradbiomed.2013.07.012, 2013.