the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Coat-colour-related genotypes, phenotyping and biometric assessment of three ecotypes of pigs in Cameroon
Aveniol Idelo Nono Ekane
Ferdinand Ngoula
Blaise Arnaud Hako Touko
A study was conducted from June 2022 to December 2023 in three agroecological zones in Cameroon to describe the local pigs and to develop a standard method for reporting their phenotypic-diversity information. A systematic sample of 152 adult pigs was considered and described in terms of coat colour major genes and body measurements. The results show that local pigs are heterogeneous in colour and are predominantly white (34.2 %). The melanocortin 1 receptor gene (MC1R) was the most significant gene for primary coat colour, with five alleles, including E+ (wild type), E (dominant black), Ed (black with white belt), Ep (white with black spots) and e (recessive red). The Ep allele was the most predominant at 75 % allele frequency. The agouti signalling protein (ASIP) gene was the only secondary coat colour determiner observed, with its recessive non-agouti allele (a) present (100 %). The coat colour modifier is the tyrosine-kinase protein gene (KIT), with six alleles, including I (colour inhibition), Id (green), Ip (white and black spots), Ib (white belt), im (dirty white) and i (non-kit), with the predominant allele being Ip (39.5 %). The agroecological zone influenced body measurements, including head length, between-ear distance, snout size and ischium width (P < 0.05). Furthermore, the highest live weight of 82.47 ± 23.86 kg was obtained in the monomodal-rainfall forest (MRF) zone. The biometrical structure shows that the local Cameroon pigs can be organised into three subgroups. This study provides the broader audience with valuable insights into the phenotypic diversity of the local Cameroon pigs and a standardised description and reporting protocol.
- Article
(2628 KB) - Full-text XML
-
Supplement
(695 KB) - BibTeX
- EndNote
The success of agriculture and livestock farming in most countries largely depends on utilising local resources to increase production. These resources have unique characteristics that are not commonly found elsewhere. Regarding livestock farming, sociological factors such as consumer preference for the colour of an animal's coat or plumage play a significant role in animal protein consumption. For instance, in Korea and many other Asian countries, consumers prefer coloured pigs over uncoloured ones (Choy et al., 2002). This makes the colour of an animal's coat critical in identifying specific breeds or crosses, similarly to blood and biochemical or molecular markers. As a result, there is a renewed interest in coat colour variations as they can help identify certain breeds or specific crosses (Legault and Chardon, 2000).
In Cameroon, the pig industry is the third largest sector of animal production, contributing over 53 877 t of meat (INS, 2019). Pigs are highly adaptable to various management and feeding conditions, and they proliferate, reach sexual maturity early, and have high prolificacy and efficient feed conversion. These qualities make them a popular choice as a livestock species (Taverner and Dunkin, 1996). Locally, the Cameroonian pig has gained recognition for its low fat percentage and disease resistance. However, the need for knowledge about this genetic resource makes it difficult for researchers, breeders and consumers to choose the best option. A phenotypic description based on the significant colouration genes could significantly benefit these users. Certain aptitudes and diseases have been linked to the colouration (Jonathan, 2003; Hutu et al., 2020; Hutt, 2022).
It is widely believed that most domestic pig breeds are black because they carry a recessive allele called mono agouti (a) at the agouti locus and a normal or wild allele (E) at the extension locus. This theory suggests that the Large White boar and wild boar have the following genotypes at the dominant white, agouti and extension loci: I/I, a/a and EP/EP and i/i, A/A and E+/E+, respectively (Ollivier and Sellier, 1982). Describing animal resources based on phenotype and genotype is efficient and cost-effective. In Cameroon, the socio-economic and phenotypic characterisation of local pig breeds has been carried out in various regions, including the Sudano–Sahelian region, humid forests with bimodal rainfall, the Western Highlands, the Sudano Guinea zone and humid forests with monomodal rainfall (Motsa'a et al., 2017; Ghomsi et al., 2022; Siewé et al., 2021). However, some unique pig ecotypes, such as the Dimako pig in humid forests with bimodal rainfall, have not been considered despite the morphological and biometrical description achieved so far. Moreover, without a standard description and reporting protocol, transferring information on genetic variability is challenging for multi-allelic genes because of the high number of gene combinations resulting in phenotype variability. This is also true for pig populations, where three genes (MC1R, ASIP and KIT) have four to seven alleles each and interact with three reported gene dilutions of two to three of these (Fontanesi and Russo, 2013).
The present study was carried out to remedy this shortcoming. Its main aim is to contribute to a better understanding and reporting of animal genetic resources and to provide a standard description and reporting protocol to a broad scientific audience.
2.1 Field study location
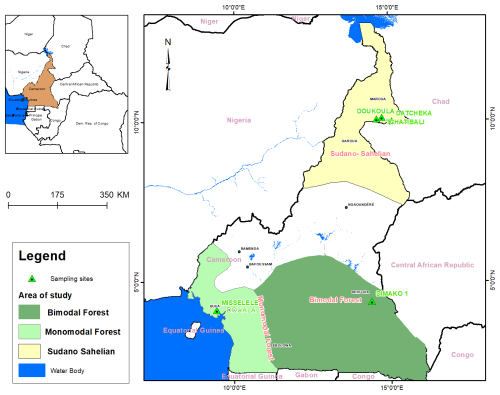
The study was conducted in Cameroon from June 2022 to December 2023 in three agroecological zones: the bimodal-rainfall forest zone (BRZ), the monomodal-rainfall forest zone (MRZ) and the Sudano–Sahelian zone (SSZ) (Fig. 1). Based on WorldClim data (2024), the SSZ is the hottest zone, with 7 to 9 months of dry season and a maximum of 5 months of rainy season. Relative humidity varies from low to high (20 % to 80 %). It is situated between 8°3′′ and 12°5′′ N and 12°3′′ and 15°4′′ E. The rainfall intensity varies from 350 to 800 mm annually, with August being the rainiest month (195 mm of rainfall). April is the hottest month (26 to 40 °C). The BRZ is located between 2°′′ and 4°5′′ N and 10°3′′ and 16°1′′ E. It is characterised by a long rainy season of 10 months, from early February to mid-December, with two peaks in May (118 mm rainfall) and September (197 mm rainfall) and with 1560 mm yearly. The dry season is short (merely 2 months). The annual temperature ranges from 17 to 32 °C, with February being the hottest month (19–32 °C). The relative humidity varies from 67 % to 87 %. The MRZ is located between 44°10′00′′–5°50′00′′ N and 9°30′00′′ E. Annual rainfall is high (2500 to 4000 mm), with July being the rainiest month (700 mm in Buea). The dry season is 3.3 months, from January to April, with February being the hottest month (26 to 32 °C). Relative humidity is high throughout the year (69 % to 87 %).
2.2 Animal material
The local pig populations of Cameroon are under-exploited and not numerically abundant, representing less than 10 % of the national pig population, which is dominated by commercial foreign breeds. They are small-sized animals mostly reared extensively in low-input systems due to their small-flock-size families (3 to 20 pigs), with sows having a litter size ranging from one to five. There is limited to no information on characteristic such as weight or housing, making it difficult to carry out characterisation studies (Motsa'a et al., 2021). The sample used was heterogeneous in terms of coat colours and was made up of adult pigs of both sexes, aged 8 months and more. Gestating sows were not sampled, and for genetically related pigs (pigs sharing an identical sire and dam), only one pig per coat colour type was considered.
2.3 Sampling and data collection
A sampling was conducted under the facilitation of the extension services of the Ministry of Livestock, Fisheries and Animal Industries (MINEPIA) to identify all possible variants of local pigs based on coat colour in each of the three agroecological zones. The sampling involved selecting 152 adult pigs representing 10 % of the pigs owned by 52 households rearing local pigs as their secondary or tertiary activity.
2.3.1 Phenotyping and identification code system
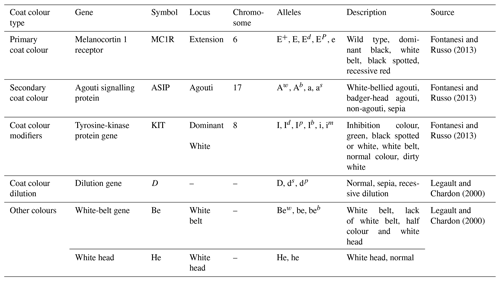
Phenotyping consisted of describing a pig based on identified significant gene loci, allelic series and visible trait characteristics. Different combinations of the major genes of a phenotype were named “morphotypes”. Each major gene was identified from its expression, and the chromosome number and corresponding locus information were obtained from the NCBI databank or credible literature sources. The major gene variants considered for the description of the morphotypes were those relating to coat colour (enhancers, dilutions and modifiers) according to Ollivier and Sellier (1992). The phenotyping was done according to Fontanesi and Russo (2013).
The characteristics of the major genes, the locus involved, their alleles, and the expressed coat colour and modifications are presented in Table 1. The coding of the phenotypic and genetic information was done according to a CL-5N system, where
-
C is the country initial (CMR for Cameroon);
-
L is the locality initial, with SSZ representing the Sudano–Sahelian zone, BRZ representing the bimodal-rainfall forest, and MRZ representing the monomodal-rainfall forest;
-
the first N is the most expressed gene allele of the primary coat colour in the phenotype (E+, E, Ep, Ed, e);
-
the second N is to be maintained if the secondary coat colour is absent or replaced by the corresponding gene initials (Aw, Ab, a, as);
-
the third N is to be maintained if the coat colour modifier is absent or replaced by gene alleles of the modifier (I, Id, Ip, Ib, I, im);
-
the fourth N is to be maintained if the coat colour enhancer or dilution is absent or replaced by gene alleles of the enhancer or dilution (D, ds, dp);
-
the fifth N is to be maintained if the coat colours of white belts or white heads are absent or replaced by the corresponding alleles (Bew; be; beb or He; he).
2.3.2 Biometric assessment and genetic frequency estimation
The biometric measurements were obtained using a graduated metre according to the protocol described by FAO (2013). A total of 19 body measurements were then assessed, as illustrated in Fig. 2.
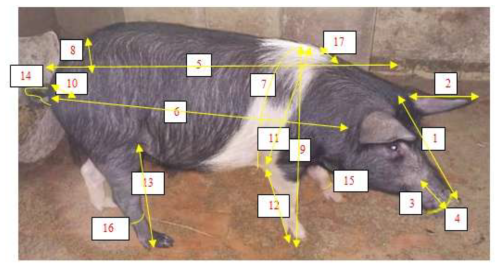
Figure 2Biometric measurements of the local pig breeds. Head length (1), ear length (2), muzzle length (3), muzzle circumference (4), body length (5), scapuloischial length (6), chest circumference (7), wither height (8), hip width (9), ischium width (10), wither height (11), front leg length (12), hind leg length (13), tail length (14), cannon circumference (15), ham circumference (16), hair length (17).
The live body weights were determined from the chest circumference, which is best predictor according to Santolini (2004), using the following barometric equation:
with Y being the body weight, X being the chest circumference, and R2 being the determination coefficient or precision.
The frequencies of the various phenotypes, genotypes and alleles were estimated as follows:
2.4 Data analysis
Descriptive statistics were used to calculate the various measurements' means, standard deviations and coefficients of variation. An analysis of variance was conducted to investigate the impact of the ecotype, coat colour and genetic type on measurements. Pearson's correlation coefficients were utilised to evaluate the degree and significance of correlation between the measurements. Principal component analysis (PCA) was performed based on the measurements to identify the main criteria explaining the morphometrical variability. A discriminant factor analysis (DFA) based on 17 body measurements, coupled with the hierarchical ascending classification (HAC), was conducted to determine the clusters of individuals in the population according to their resemblance to their biometrical measurements. The statistical analyses were conducted using SPSS 21.0 and XLSTAT 2022 software.
3.1 Phenotyping of local pigs in the three agroecological zones of Cameroon
3.1.1 Frequency distribution of coat colours
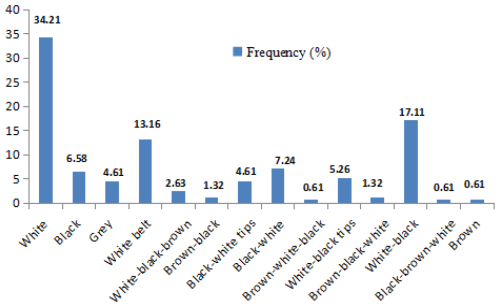
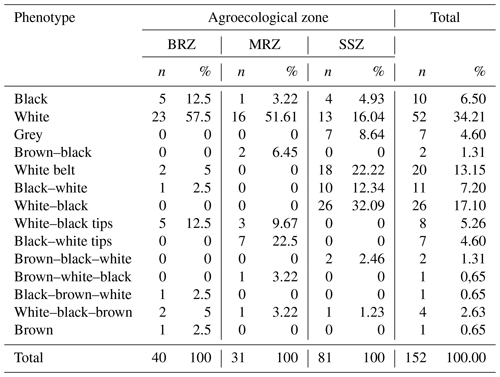
The frequency distributions of the coat colours of local pig morphotypes are presented in Fig. 3. The most common uniform colour pattern is white (34.21 %), followed by black (6.58 %), grey (4.61 %) and brown (0.65 %). The most usual colour pattern combinations are white–black (17.11 %), followed by black with a white belt (13.16 %) and eight other combinations (23.79 %).
3.1.2 Morphotype description and coding
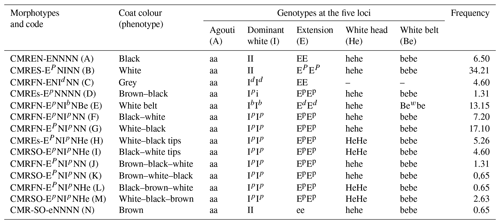
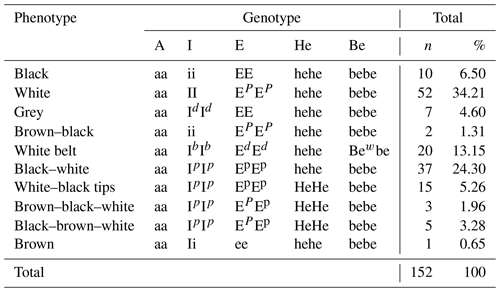
The phenotypes and the corresponding genotypes and CL-5N codes of pigs for the coat colour genes are presented in Table 2. A total of 14 morphotypes are described, with 3 genes being responsible for primary (MC1R) and secondary coat colours (ASIP and KIT). The MC1R gene identified has four allele series present (E, Ep, Ed and e) out of the four known so far. The ASIP gene has two (A, a) out of the four known so far, and the KIT has four (I, Id, Ib and i) out of the six known so far. Two gene modifiers are responsible for the white coat colour pattern (white belt and white head). The extension locus of the MC1R gene is the primary coat colour gene observed in all the pigs, either being homozygote dominant (EE) and associated with spotting (EpEp), having a white belt (EdEd), or being heterozygotic due to the recessive allele form (e).

Figure 4Picture and morphotype code highlighting the genes for primary, modifier and other feathering colours in pigs at the MC1R locus, agouti locus and extension locus. Shown for the MC1R: E (dominant black), Ed (black with white belt), Ep (black-spotted white) and e (recessive red). The modifier is the tyrosine kinase protein gene (I for colour inhibition, Id for green, Ip for white and black spots, and Ib for the white belt). Also shown are the white belt (Bew) and the white head (He). Also shown: the white belt (Bew) and white head (He).
Of the 14 morphotypes identified (Table 2), 7 are located in the far north, 3 are located in the east, and 4 are located in the southwest. It can be noticed that different morphotypes may have the same colour combinations but in various proportions, dilutions and distributions, as observed in the morphotypes J, K, L and M, which are all brown, white and black colour combinations. The morphotype pictures and the corresponding standard coding system are presented in Fig. 4.
3.1.3 Phenotype, genotype and allele frequencies of coat colour genes
3.1.4 Phenotype frequency
Table 3 shows the frequency distribution of the phenotypes of the local pigs in the three agroecological zones of Cameroon. The white pig is the most common, with the highest frequency observed in the BRZ (57.5 %) and then in the MRZ (51.16 %) and the SSZ (16.4 %). The black pig comes second among monochromes, with 12.5 % in the BRZ and then 4.96 % in the SSZ and 3.22 % in the MRZ. The grey pig was only present in the SSZ (4.60 %), whereas the brown pig was observed only in the BRZ (2.5 %). The most common colour combinations included the black–white-belt phenotype at 22.22 % and 2.5 % in the SSZ and BRZ, respectively, followed by the white–black phenotype (32.69 %) observed only in the SSZ.
3.1.5 Genotype frequency
Table 4 presents the genotype frequency of the indigenous pigs of Cameroon for the five loci of interest. Only 10 genotypes were obtained for the 14 phenotypes as four different morphotypes presented the same genotype, including black–white (F) and white–black (G); white–black tips (H) and black–white tips (I); brown–black–white (J) and brown–white–black (K); and, finally, black–brown–white (L) and white–black–brown (M). The most frequent genotype (aa/II/EPEP/hehe/bebe was monochrome white (34.21 %). The least frequent genotype (aa/ii/Ee/hehe/bebe) was monochrome brown (0.65 %).
3.1.6 Allele frequency
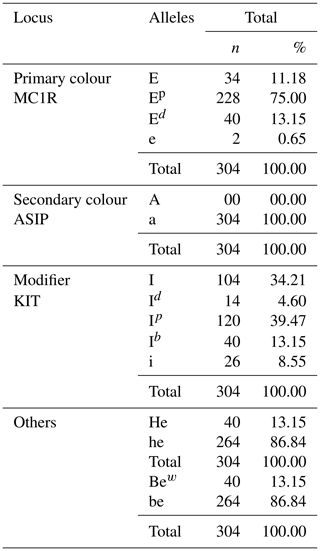
Table 5 shows the allele frequency of the local pig population. A total of 12 alleles of coat colour and modifiers were observed, including 4 alleles for the primary coat colour at the MC1R locus, 1 allele for secondary colours at the ASP locus, 4 alleles for modifiers at the KIT locus and finally 4 alleles for colour enhancers or dilutors at undescribed loci. The allele with the highest frequency is Ep (75.00 %), and the allele with the lowest frequency is e (0.65 %). The melanocortin 1 receptor (MC1R) locus is the most determining coat colour gene, with four alleles present (E, Ep, e and Ed) at 11.18 %, 75.00 %, 0.65 % and 13.15 %, respectively.
3.2 Biometrical characteristics of the local pigs of Cameroon
3.2.1 Body measurements of local pigs of Cameroon
Table 6 shows that most of the biometric measurements were influenced by the agroecological zone (P < 0.05), except for the length of the head, the length of the snout and the ischium width (P > 0.05). Furthermore, the highest live weight (82.47 ± 23.86 kg) was obtained in the MRZ. The coefficient of variation suggests that the population of local pigs in Cameroon is relatively dispersed for almost all 17 variables.
Table 6Biometric characteristics (mean and coefficient of variation) of local pig populations in Cameroon according to agroecological zone.
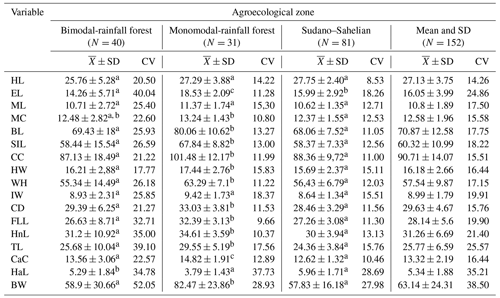
a, b, c, d The values which have the same letter in the same column were comparable statistically (P < 0.05). Head length is denoted by HL, head height is denoted by HH, ear length is denoted by EL, ear width is denoted by EW, muzzle length is denoted by ML, muzzle circumference is denoted by MC, body length is denoted by BL, scapuloischial length is denoted by SIL, chest circumference is denoted by CC, hip width is denoted by HW, wither height is denoted by WH, ischium width is denoted by IW, chest depth is denoted by CD, front leg length is denoted by FLL, hind leg length is denoted by HnL, tail length is denoted by TL, cannon circumference is denoted by CaC, ham circumference is denoted by HC, hair length is denoted by HaL, and body weight is denoted by BW.
3.2.2 Correlation between body measurements
Table 7 shows the correlation between body measurements. Strong and weak correlations were observed here and there, being either positive or negative, with most of them being significant at 5 %. The strongest (r=0.9035) and most significant (P < 0.05) one was found between body length (BL) and scapula ischial length (SIL).
Table 7Correlation matrix (Pearson (n)) between measurements of local pigs in Cameroon.
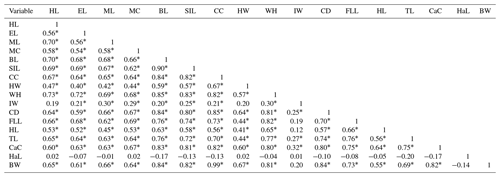
∗ The correlation is significant at the level of 0.01. Head length is denoted by HL, head height is denoted by HH, ear length is denoted by EL, ear width is denoted by EW, muzzle length is denoted by ML, muzzle circumference is denoted by MC, body length is denoted by BL, scapuloischial length is denoted by SIL, chest circumference is denoted by CC, hip width is denoted by HW, wither height is denoted by WH, ischium width is denoted by IW, chest depth is denoted by CD, front leg length is denoted by FLL, hind leg length is denoted by HnL, tail length is denoted by TL, cannon circumference is denoted by CaC, ham circumference is denoted by HC, hair length is denoted by HaL, and body weight is denoted by BW.
3.2.3 Phenotype variability of local pig ecotypes
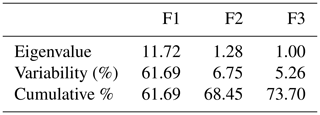
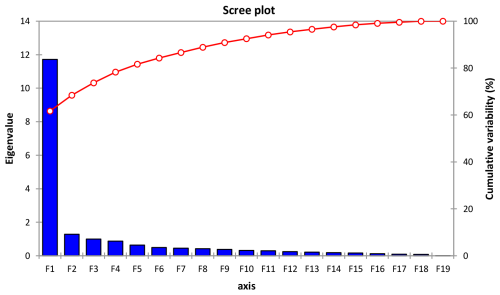
From Table 8 and Fig. 5, 3 components out of 19 explain most of the variability at 61.69 %, 6.75 % and 5.25 % for a cumulative variability of 73.70 %. These percentages correspond to eigenvalues of 11.72, 1.28 and 0.99. This information implies that if data are represented on two axes then 73.70 % of the total variability will be preserved. For the F1 factor, four variables mostly contributed above 7 % each to the observed variability, including, by order of importance, body length (7.37), wither height (7.28), chest circumference (7.15) and cannon circumference (7.11) (Table S1 in the Supplement).
3.2.4 Biometric structure of local pig population
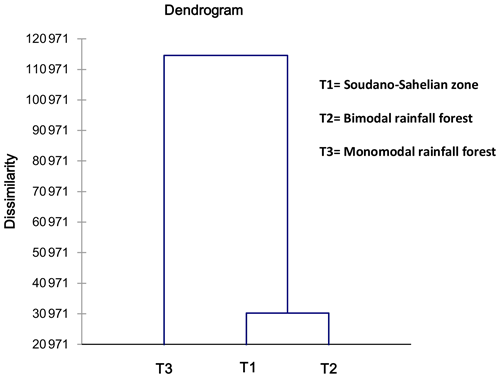
Figure 6 is the dendrogram of the biometric structure of the local pigs from the three agroecological zones based on dissimilarity in terms of Euclidean distance using Ward's method. The dendrogram presented three subpopulations (T1, T2, T3).
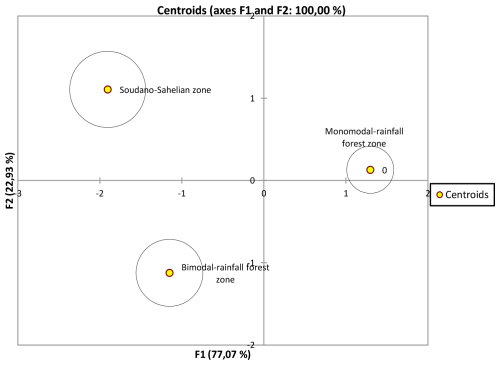
Figure 7Discriminate analysis representation of F1 and F2 axes of T1, T2 and T3 based on their barycentre distance.
It clearly appears to be the fact that T1 and T2 are not similar but are closer to each other compared to T3 (Table 9 and Fig. 6). The discriminate analysis confirmed a differentiation of the three subpopulations (Fig. 7) displayed according to the F1 and F2 axes, with significant admixture evidenced among T1, T2 and T3 (Fig. S1 in the Supplement).
Table 9Distance between the barycentres of agroecological zones.
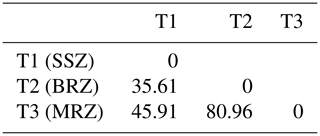
BRZ denotes the bimodal-rainfall forest zone, MRZ denotes the monomodal-rainfall forest zone, and SSZ denotes the Sudano–Sahelian zone.
A lower variance (30.79 %) is observed within a population, suggesting an important level of breed homogeneity or selection. The higher between-population variance component (69.21 %) indicates a higher polymorphism rate among the three pig subpopulations of Cameroon.
This study revealed a great variety of coat colours as an expression combination of primary and secondary coat colour genes, as well as of modifiers and dilutors. The great variety of coat colours has been also evidenced by Djimenou et al. (2018), Siewe et al. (2021), Ghomsi et al. (2022) and Zhong et al. (2022), respectively, in Benin, Cameroon, Cameroon and China. However, this study is the first of it kind with regard to the phenotyping and coding of the phenotypic-diversity information as previous literature on this topic has not been found. Earlier reports from our predecessors revealed that the native pig from Cameroon is primarily black in terms of coat colour (Motsa'a et al., 2017). Therefore, the diversity in terms of coat colours observed in this study suggests that local pigs in Cameroon may have benefited from crosses with exotic breeds, including Black Hampshire, Berkshire, Yorkshire, Large White, Landrace, Piétrain and Duroc, as confirmed by their coat pattern characteristics in the described samples. This statement is in agreement with the report of AU-BIRA (2016), in which it was stated that cross-breeding of local and European breeds was the common genetic improvement practice across Africa, including in Cameroon. As evidence, crossbreeding of Black Hampshire, Large White and local pigs from the Bamboutos resulted in the black and white Bamileke pig, with white patterns from the Large White breed. Furthermore, the Sudano–Sahelian pig was obtained by crossing two exotic breeds, namely Yorkshire and Berkshire, with the indigenous pig of the far north (Mopate et al., 2006).
Coat colour patterns influenced by major genes can influence the production performance of pig breeds. In this regard, evidence suggests that coat colour genes have an impact on production and reproduction traits, with white pigs showing good reproductive performance (Li et al., 2022). On the other hand, the dwarf pig of Ghana, characterised by its black coat colour, is endangered, with exceptional hardiness and disease-resistant merit (Amponsah et al. 2017). Despite these reports on the correlation between white coats and reproductive traits and white coats and hardiness, more investigations are needed to confirm these conclusions as the white coat colour could also have been a choice in selective breeding for reasons of aesthetics and market trends guided by consumer preferences.
The high proportion of white coats (34.21 %) could be justified for two reasons. Primarily, it could be the result of the introgression of Large White or Landrace breeds into local pig populations for their reproduction- and growth-based genetic merits in relation to the rest of the studied population. Secondly, it may suggest that Cameroonian consumers prefer white pigs. This contradicts the assertions by Choy et al. (2002), who state that, in Korea, as in many other Asian countries, consumers prefer coloured pigs to monochrome ones.
A total of 14 morphotypes have been described, and a CL-5N coding system has been developed for information sharing with regard to phenotype diversity in pigs. This study cannot pretend to have exhausted the existing morphotype in Cameroon due to the limited sample size per zone and the limited study coverage (three zones out of five). The strong involvement of the MC1R alleles (75 %) in the colour determinism of Cameroon pigs converges with the assertions of many authors that MC1R is one of primary major genes regulating the synthesis of eumelanin (black and brown) and phaeomelanin (red and yellow) in mammalian melanocytes (Fontanesi and Russo, 2013; Legault and Chardon, 2000; Kijas et al., 1998). The Ep (0.75) allele of the MC1R gene has a higher frequency than that obtained by Carrion et al. (2003) in Iberian pigs (0.41), while the e (0.00) allele of the MC1R has a lower frequency compared to that obtained by the above-mentioned authors (0.31). This is explained by the fact that those alleles are characteristic of Iberian pigs, notably white, spotted and red pigs, and, therefore, constitute the origin of the introgression of both alleles into Cameroonian pig populations. The predominance of white and red coat colours in Iberian pigs is confirmed by Zhong et al. (2022).
Coat-colour-related genotypes are mendelian traits with a visible effect on the phenotype. This made it easy to identify the existing genotypes of the local pigs of Cameroon. Different combinations of the same colour patterns were observed. For example, different combinations of black, white and brown colours were observed in J, K, L and M (Table 2 and Fig. 4). Hur et al. (2013) observed white-spotted black coats and black-spotted white coats as two combinations of black and white resulting from the crossing of pure-black Korean native pigs and white Landrace pigs. This evidence shows that both combinations are rooted in the same coat-colour-based genotype. The difference in terms of their distribution across the body is probably the result of the difference in coat colour gene interactions. Fontanesi (2022) reported that pig breeds can be determined by the combination of hair colour, skin colour, and the bodily position of the pigmented regions of hair and skin. Therefore, a detailed description of genes and their mutations affecting coat colour and pigmentation in pigs will be a step toward selective breeding in Cameroon.
An analysis of biometric data was carried out and compared with the analyses of other authors. It was found that the head length results of this study were greater than those of Siewe et al. (2021) and Adeola et al. (2013), whereas the ear length, body length and muzzle length were lower than those recorded by Siewe et al. (2021) and greater than those of Adeola et al. (2013). The structuring of the local pigs of Cameroon into subgroups according to their agroecological zones as done in this study matches with the studies of Motsa'a et al. (2021) and Djimenou et al. (2018) on local pigs from Cameroon and Benin, respectively. The studied pig population can therefore be considered to be made up of distinct ecotypes based on their biometric structure as influenced by environmental factors.
The level of introgression of the Ep allele responsible for the white coat colour and black spots in pigs bred in Cameroon, with a frequency of 75 % in the studied subpopulations, is sufficient evidence that the local breed is under threat of genetic absorption since this allele is typical of commercial pigs such as the Large White, Landrace, Piétrain and Berkshire breeds, which are exotic breeds. This observation concurs with that in the report of AU-BIRA (2016) which states that local African breeds are constantly threatened by genetic erosion phenomena.
The aim of this study was to describe the local pigs of Cameroon and to develop a standard method for reporting phenotypic-diversity information. The study revealed that pigs bred in Cameroon have a multitude of coat colours, reflecting crossbreeding with commercial breeds. In addition, the MC1R gene is the primary coat colour determiner and plays a major role in the coat colouration of pigs bred in Cameroon. The agroecological zone (environmental factor) influences the biometric structure of the local pigs of Cameroon, separated into subgroups or ecotypes. The CL-5N coding system developed in this study is innovative and will standardise phenotypic information sharing within the scientific community and between stakeholders for a harmonised record system of genetic information, breed reporting and conservation information.
The data presented in this study are available upon request from the corresponding author.
In this study, samples were not collected from animals in the field. The phenotype descriptions were obtained through the cooperation and consent of the farmers, ensuring ethical access to the animals involved.
The supplement related to this article is available online at https://doi.org/10.5194/aab-68-239-2025-supplement.
Conceptualisation was done by HTBA, NEAI and NF. The methodology was the responsibility of NEAI, HTBA and NF. Data collection was done by NEAI. Data analysis was done by HTBA and NEAI. Drafting of the paper was done by NEAI. Review and editing were done by HTBA and NF. The research was jointly supervised by HTBA and NF. All the authors read and approved the paper.
The contact author has declared that none of the authors has any competing interests.
The research was carried out under the supervision of the Coordinator of the Biotechnology and Bioinformatics Research Unit following the CCAC guidelines on: the care and use of farm animals in research, teaching and testing (CCAC, 2009). All of the blood samples were collected, and the data were analysed strictly following these guidelines.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors are grateful to all the staff of the Regional Delegation of Livestock, Fisheries and Animal Industries of the regions of the far north, east, and southwest for the facilitation of interviews and fieldwork. We also thank the pig farmers for their availability during the investigation.
This paper was edited by Henry Reyer and reviewed by two anonymous referees.
Adeola, A. C., Saidu, O. O., and Ofelia, G. O.: Morphological characterization of indigenous and crossbred pigs in rural and peri-urban areas of southwestern Nigeria, J. Anim. Sci., 3, 230–235, 2013.
Amponsah, R. O., Skinner, B. M., Adjei, D. O., Bauer, J., Larson, G., Affara, N. A., and Sargent, C. A.: Origin and phylogenetic status of the local Ashanti Dwarf pig (ADP) of Ghana based on genetic analysis, BMC Genomics, 18, 1–12, 2017.
AU-BIRA: Le porc local africain in Cannaissez vos animaux, Bureau InterAfricain des Ressources Animales, Union Africaine, 1, 30–34, 2016.
Carrion, D., Day, A., Evans, C., Mitsuhashi T., Archibald, A., Haley, C., Andersson, I., and Plastow, G.: The use of MC1R and Kit genotypes for breed characterisation, Arch. Zootec., 52, 237–244, 2003.
CCAC (Canadian Council on Animal Care): CCAC Guidelines on the Care and Use of Farm Animals in Research, Teaching and Testing, Canadian Council on Animal Care, https://ccac.ca/Documents/Standards/Guidelines/Farm_Animals.pdf (last access: 17 February 2024), 2009.
Choy, Y. H., Jeon, G. J., Kim T. H., Choi, B. H., and Chung, H. W.: Ear Type and Coat Color on Growth Performances of Crossbred Pigs, J. Anim. Sci., 15, 1178–1181, 2002.
Climate World Data: The climate of Cameroon, WorldData.com, https://www.donneesmondiales.com/afrique/cameroun/climat.php (last access: 20 March 2025), 2024.
Djimènou, D., Adoukounou, S. H., Chrysostome, C., and Koudande, O. D.: Caractérisation phénotypique des porcs locaux (Sus scrofa domesticus) au Sud du Bénin, J. Anim. Plant Sci., 37, 5956–5975, http://www.m.elewa.org/JAPS (last access: 20 March 2025), 2018.
FAO: Phenotypic characterization of animal genetic resources. FAO Guidelines on Animal Production and Health, 11, Rome, FAO, ISBN 978-92-5-107199-1, https://www.fao.org/4/i2686e/i2686e00.pdf (last access: 20 March 2025), 2013.
Fontanesi, L.: Invited review: Genetics and genomics of pigmentation variability in pigs: A review, Livest. Sci., 265, 105079, https://doi.org/10.1016/j.livsci.2022.105079, 2022.
Fontanesi, L. and Russo, V.: Molecular genetics of coat colour in pigs, Acta Agr. Slov., 4, 15–20, 2013.
Ghomsi, O. S. M., Wirnkar, C. N., Etchu, K. A., Fontanesi, L., Bilong, C. F. B., and Moundipa, F.: Morphological and serum biochemical characterizations of local pig populations from three different agro-ecological areas of Cameroon, Int. J. Livest. Prod., 13, 33–42, 2022.
Hur, S. J., Jeong, T. C., Kim, G. D., Jeong, J. Y., Cho, I. C., Lim, H. T., Kim, B. W., and Joo, S. T.: Comparison of live performance and meat quality parameter of cross bred (korean native black pig and landrace) pigs with different coat colors, Asian Austral. J. Anim., 26, 1047–1053, https://doi.org/10.5713/ajas.2013.13005, 2013.
Hutt, F. B.: Genetic indicators of resistance to disease in domestic animals, Department of poultry Science, Cornell University Agricultural Experiment station, Ithaca, New York 14850, United States, 179–185, 2022.
Hutu, I., Oldendroek, K., and Zaaij L. V. D.: Élevage et amélioration génétique des animaux: Caractéristiques morphologiques et productives des animaux domestiques, Agroprint, Timisoma, Koumani, https://doi.org/10.13140/RG.2.2.11939.99366, ISBN 978-606-785-148-9, 2020.
INS: Annuaire Statistique du Cameroun, 11 pp., https://ins-cameroun.cm/wp-content/uploads/2021/02/0CHAPITRE-14_PECHE-ET-ELEVAGE.pdf (last access: 20 February 2025), 2019.
Jonathan, L. R.: Genetics of hair and skin colour, Annu. Rev. Genet., 37, 67–90, https://doi.org/10.1146/annurev.genet.37.110801.143233, 2003.
Kijas, J. M. H., Wales, R., Tornsten, A., Chardon, P., Moller, M., and Andersson, L.: Melanocortin Receptor 1 (MC1R) Mutations and Coat Color in Pigs, Genetics, 150, 1177–1185, 1998.
Legault, C. and Chardon, P.: Génétique de la coloration de la robe chez le Porc, Journées de la Recherche Porcine, 32, 385–395, 2000.
Li, Y., Yuan, R., Gong, Z., Zou, Q., Wang, Y., Tang, G., Zhu, L., Li X., and Jiang, Y.: Evaluation of coat color inheritance and production performance for crossbreed from Chinese indigenous chenghua pig crossdred with Berkshire, Anim. Biosci., 35, 1479–1488, 2022.
Mopaté, L. Y., Koussou, M. O., and Kaboré, Z. C. Y.: L'élevage porcin au Tchad: bilan de l'introduction, de l'amélioration et de la diffusion des races exotiques, AGRI, FAO, Rome, Italie, 38, 87–98, 2006.
Motsa'a, S. J.: Biodiversity of pigs (Sus scrofa domesticus) in the Humid forest with monomodal rainfall agro-ecological zone of Cameroon, MS thesis, FASA/UDS, 5–52, 2017.
Motsa'a, J. S., Defang, H. F., Hako, T. B. A., Ojong, E. T., Mube, K. H., Nguekem1, C. L., Tagning, Z. P. D., Mouchili, M., and Keambou, T. C.: Socioeconomic and productive characteristics of indigenous pig farming in Cameroon, Applied Animal Husbandry and Rural Development, 14, 32–39, http://www.sasas.co.za/aahrd/ (last access: 20 March 2025), 2021.
Ollivier, L. and Sellier , P.: Pig genetics: a review, Ann. Génét. Sél. Anim., 14, 481–544, https://doi.org/10.1186/1297-9686-14-4-481, 1982.
Santolini, J.: Impact économique d'un traitement antiparasitaire en élevage porcin traditionnel, dans le district de Tramkak, Cambodge, Rapport de stage, DESS Productions animales en régions chaudes, CIRAD-emvt, Université Montpellier, France, 63 p, 2004.
Siewe, A. T., Manjeli, Y., and Meutchieye, F.: The Bankim Pigs: A Native Cameroonian, Genetics and Biodiversity Journal, 5, 101–111, 2021.
Taverner, M. R. and Dunkin, A. C. (Eds.): Introduction to pig production, in: Pig Production, Elsevier Science, the Netherlands, ISBN 0444883479, 1996.
Zhong, H., Zhang, J., Tan, C., Shi, J., Yan, J., Cai, G., Wu, Z., and Yang, H.: Pig coat colour manipulation by MC1R gene editing, Int. J. Mol. Sci., 23, 10356, https://doi.org/10.3390/ijms231810356, 2022.