the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Association analysis of melanophilin (MLPH) gene expression and polymorphism with plumage color in quail
Zhiwen Yuan
Xiaohui Zhang
Youzhi Pang
Yanxia Qi
Qiankun Wang
Yunqi Hu
Yiwei Zhao
Shiwei Ren
Linke Huo
We explore the relationship between the melanophilin (MLPH) gene and quail plumage color and provide a reference for subsequent quail plumage color breeding. In this experiment, real-time quantitative PCR (RT-qPCR) technology was used to analyze the relative mRNA expression levels of Korean quail (maroon) and Beijing white quail embryos at different developmental stages. Two single-nucleotide polymorphisms (SNPs) in the MLPH gene were screened based on the RNA-sequencing (RNA-Seq) data of skin tissues of Korean quail and Beijing white quail during the embryonic stage. Kompetitive allele-specific PCR (KASP) technology was used for genotyping in the resource population, and correlation analysis was carried out with the plumage color traits of quail. Finally, bioinformatics was used to predict the effects of these two SNPs on the structure and function of the encoded protein. The results showed that the expression level of the MLPH gene during embryonic development of Beijing white quail was significantly higher than that of Korean quail (P<0.01). The frequency distribution of the three genotypes (CC, CA and AA) of the Beijing white quail at the c.1807C > A mutation site was significantly different from that of the Korean quail (P<0.01). The frequency distribution of the three genotypes (GG, GA and AA) of the Beijing white quail at the c.2129G > A mutation site was significantly different from that of the Korean quail (P<0.01). And there was a significant correlation between the c.1807C > A mutation site and the white plumage phenotype. Bioinformatics showed that SNP1 (c.1807C > A) was a neutral mutation and that SNP2 (c.2129G > A) was a deleterious mutation. The prediction of protein conservation showed that the mutation sites of coding proteins R603S and G710D caused by SNP1 (c.1807C > A) and SNP2 (c.2129G > A) were highly conserved.
- Article
(2351 KB) - Full-text XML
- BibTeX
- EndNote
Plumage color is an important appearance and quality trait of poultry, which can be used not only for variety identification but also for molecular genetic breeding markers (Mastrangelo et al., 2020). In poultry genetic breeding, plumage color has always been an important breeding indicator, which is closely related to consumer preferences and has important economic value (Xi et al., 2020). Plumage formation is closely related to melanin deposition and degradation. Avian plumage color differences are mainly due to the uneven distribution of melanin in the body. Melanin is divided into eumelanin and pheomelanin. As the main component of avian plumage color formation, melanin deposition and degradation will have an important impact on the formation of plumage color (Nam et al., 2021; Li et al., 2012). Melanin synthesis is mainly by amino acid synthesis of tyrosine in the body, through the tyrosinase catalytic body tyrosine hydroxylation and a series of biochemical reactions (Raghunath et al., 2015; Galeb et al., 2021). Once functional disorder occurs in these processes, it will lead to melanin synthesis defects, which will lead to abnormal pigmentation (Wakabayashi et al., 2021). Quail is an important avian animal, and the genetic mechanism of plumage color is very complex. Studies have shown that TYR, TYRP1, MC1R and ASIP genes are related to the formation of plumage color in quail (Li et al., 2019; Xu et al., 2013).
The melanophilin (MLPH) gene is a Rab effector protein involved in melanosome transport, which can regulate skin pigmentation during melanosome transport (Manakhov et al., 2019; Myung et al., 2021). As a protein complex involving myosin Va (MyoVa) and Rab27a, MLPH protein also enables the movement and migration of melanin bodies in melanocytes (Lee et al., 2019). Studies have shown that MLPH mutations affect the transport of melanin from the skin to the hair follicles, leading to the loss of melanin transport function (Zhang et al., 2020; Moravčíková et al., 2021; Doig et al., 2013). At present, studies have found that MLPH gene mutation will lead to coat color dilution in cats, dogs and rabbits (Ishida et al., 2006; Lehner et al., 2013; Bauer et al., 2018). However, the correlation between the MLPH gene mutation and avian plumage color is rare; the correlation between the MLPH gene mutation and quail plumage color is especially less reported. Studying the differences in the MLPH gene mutations in different quail populations can help us to further understand the genetic mechanism of quail plumage color.
2.1 Animals
The study protocol was approved by the Animal Care Committee of Henan University of Science and Technology. The study was conducted in the animal genetic breeding laboratory of the College of Animal Science and Technology, Henan University of Science and Technology. The study was conducted from May to September 2022. Korean quail (the color of the plumage is maroon) and Beijing white quail (the color of the plumage is white) eggs (36 each) were purchased from Quail Seed Industry Co., Ltd., Henan University of Science and Technology. The quail eggs were incubated in an incubator at (37 ± 1) ∘C with relative humidity of 50 %–65 %. Meanwhile, 15 mg samples of embryonic skin tissue were taken at days 8, 10, 12 and 14 and then frozen in liquid nitrogen for subsequent RNA extraction. In total, 350 eggs of Korean quail and 350 eggs of Beijing white quail were purchased, and the skin tissues of each embryo were collected on the 10th day of incubation and frozen in liquid nitrogen for subsequent genomic DNA extraction.
2.2 Total RNA extraction and complementary DNA (cDNA) synthesis
The skin tissue of quail embryos was crushed and ground, and the total RNA was extracted by TRIzol reagent (Invitrogen, Thermo Fisher Scientific, USA) according to the manufacturer's instructions. The RNA concentration was detected by a NanoDrop D2000 (Thermo Fisher Scientific, USA), and the RNA quality was detected by 1 % agarose gel electrophoresis. Total RNA was reverse-transcribed into first-strand cDNA using the “PrimeScript™ IV 1st strand cDNA Synthesis Mix” reverse transcription kit (TaKaRa, Japan) and stored at −20 ∘C.
2.3 Detection of MLPH mRNA expression by RT-qPCR
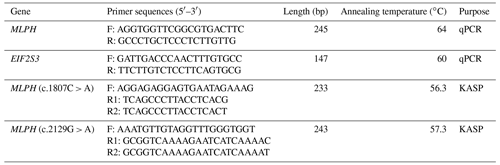
Using EIF2S3 (Yuan et al., 2022) as the reference gene, real-time quantitative PCR (RT-qPCR) was used to detect the expression level of the MLPH gene in different developmental stages of Korean quail and Beijing white quail embryos. The MLPH and EIF2S3 primers were designed by Primer 5.0 software and synthesized by Wuhan Aoke Dingsheng Biotechnology Co., Ltd. (Aoke Dingsheng, Wuhan, China). The primer information is shown in Table 1. The RT-qPCR reaction system (20 µL) consisted of 1 µL of cDNA template, 10 µL of SYBR® Premix Ex Taq™ II (2×), 0.7 µL of forward primers, 0.7 µL of reverse primers and 7.6 µL of ddH2O (double-distilled). The RT-qPCR reaction conditions were as follows: pre-denaturation at 95 ∘C for 3 min, denaturation at 95 ∘C for 20 s, annealing for 30 s (the annealing temperature of each gene is shown in Table 1) and extension at 72 ∘C for 20 s (40 cycles); fluorescence signals were collected during the extension phase. The melting curve was increased by 0.5 ∘C every 5 s from 65 to 95 ∘C.
2.4 Genomic DNA extraction and detection
The embryonic skin tissues of Korean quail and Beijing white quail were crushed and ground, and the genomic DNA was extracted by an animal genomic DNA extraction kit (TaKaRa, Japan). DNA concentration was detected by a NanoDrop D2000, and DNA quality was detected by 1 % agarose gel electrophoresis.
2.5 MLPH gene polymorphism detection
Two single-nucleotide polymorphisms (SNPs) in the MLPH gene were screened based on the RNA-sequencing (RNA-Seq) data of skin tissues of Korean quail and Beijing white quail during the embryonic stage. The gene sequence information of Japanese quail was obtained from the National Center for Biotechnology Information (NCBI) database. Two pairs of primers (one positive and two negative) were designed using Primer 5.0 software, and the primers were synthesized by Wuhan Aoke Dingsheng Biotechnology Co., Ltd. (Table 1). The MLPH genes of Korean quail and Beijing white quail were genotyped by Kompetitive allele-specific PCR (KASP) technology. The KASP reaction system conditions were as follows: KASP 2× Master mix V3 (5 µL), forward universal primer F (0.9 µL), reverse typing primer R1 (0.3 µL), reverse typing primer R2 (0.3 µL), template DNA (1 µL) and ddH2O (2.5 µL); the total volume was 10 µL. The reaction conditions were as follows: the first step was 94 ∘C for 6 min; the second step was 94 ∘C denaturation for 20 s, 61 ∘C annealing for 60 s (from the second cycle, 0.6 ∘C per cycle), 10 cycles; the third step was 94 ∘C denaturation for 20 s, 55 ∘C annealing for 60 s, 26 cycles; and step four was 37 ∘C for 5 min (KASP typing data should generally be read below 40 ∘C). The amplified products were read by a fluorescence quantitative PCR instrument (Bio-Rad, USA) to obtain the relative fluorescence unit (RFU) values of each sample. Genotyping was generated using R, version 4.1.2, ggplot2 package.
2.6 Bioinformatics of the MLPH gene encoding protein
The protein sequence of the MLPH gene was analyzed by bioinformatics. The software packages and their website information are shown in Table 2.
2.7 Statistics and analyses
The 2−ΔΔCt method was used to calculate the relative expression of the gene (Livak et al., 2001). The t test was performed by SPSS 20.0 software, and a graph was drawn by GraphPad Prism 8.0. A chi-squared test was performed by SPSS 20.0 software to evaluate the allele frequency of each SNP and its association with plumage color. Polymorphic information content (PIC) was calculated using PICcalc software (Nagy et al., 2012).
3.1 mRNA expression level of the MLPH gene
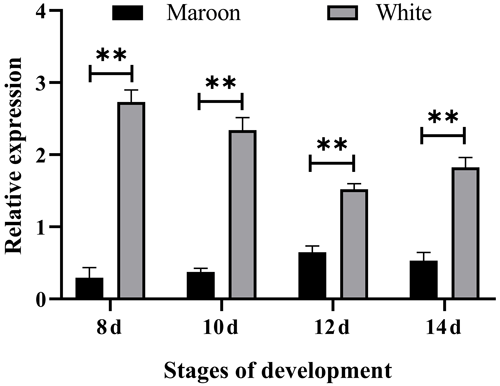
RT-qPCR results showed that the expression levels of the MLPH gene in Korean quail and Beijing white quail at different stages of embryonic development were significantly different, and the relative expression level of Beijing white quail was significantly higher than that of Korean quail (Fig. 1). This indicates that the MLPH gene inhibits melanin synthesis in quail plumage color formation.
3.2 Genotype analysis and genetic information analysis of the MLPH gene mutation sites
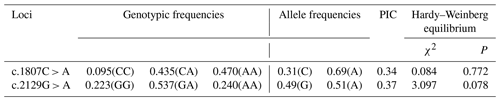
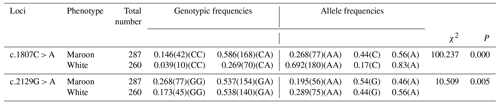
The results showed that there were three genotypes (CC, CA and AA) in exon 13 and three genotypes (GG, GA and AA) in exon 16 of the MLPH gene (Fig. 2). According to the genetic information of the MLPH gene mutation site, the dominant allele of the c.1807C > A mutation site in the quail population was A, and the dominant allele frequency was 0.69. The dominant allele of the mutation site c.2129G > A was A, and the dominant allele frequency was 0.51. By calculating the polymorphism information content of Korean quail and Beijing white quail, the two mutation sites of the MLPH gene were moderate polymorphisms (0.25 < PIC < 0.5). A Hardy–Weinberg equilibrium test on each mutation site showed that the quail population was in Hardy–Weinberg equilibrium at both mutation sites (P>0.05), as shown in Table 3.
3.3 Association analysis of the MLPH gene polymorphism with plumage color traits
The association analysis between the MLPH gene and quail plumage color traits showed that there was a significant correlation between the MLPH gene c.1807C > A mutation site and quail plumage color traits (P<0.01), where allele A in the Beijing white quail population occupies an absolute advantage, indicating that the mutation site will hinder the pigment deposition of quail plumage. The frequency distribution of the three genotypes of c.2129G > A in Beijing white quail was significantly different from that in Korean quail (P<0.01). Allele G had an advantage in Korean quail, and allele A had an advantage in Beijing white quail, indicating that the plumage color traits of maroon plumage and white plumage at this mutation site were correlated with the frequencies of alleles G and A, respectively (Table 4).
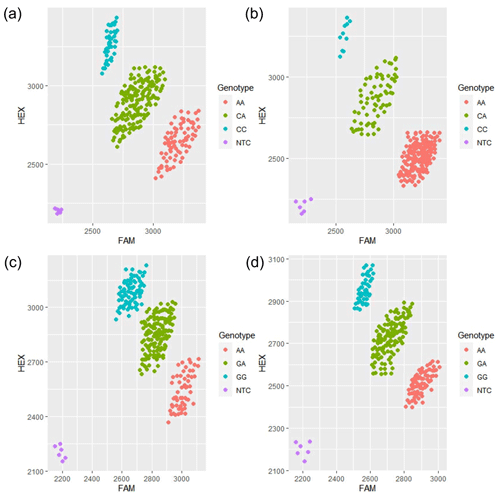
Figure 2Principal component analysis (PCA) typing diagram of each mutation site of the MLPH gene. (a) PCA graph of c.1807C > A for Korean quail; (b) PCA graph of c.1807C > A for Beijing white quail; (c) PCA graph of c.2129G > A for Korean quail; (d) PCA graph of c.2129G > A for Beijing white quail.
3.4 Bioinformatics of the MLPH protein
The analysis showed that the MLPH protein did not have the signal peptide and belonged to non-secretory proteins. The prediction results of TMHMM online software showed that the MLPH protein had no transmembrane region, and the total probability of the MLPH protein being located on the membrane cytoplasm side was 0.00078.
Three nonsynonymous SNP (nsSNP) online functional prediction software packages and one stability prediction software package were used to analyze the two nsSNP stability prediction software packages, and the results were slightly different. Comprehensive analysis showed that SNP1 (c.1807C > A) was a neutral mutation and that SNP2 (c.2129G > A) was a deleterious mutation (Table 5). The prediction results of MutPred online software showed that the R603S and G710D sites may not cause changes in membrane protein structure (Table 6).
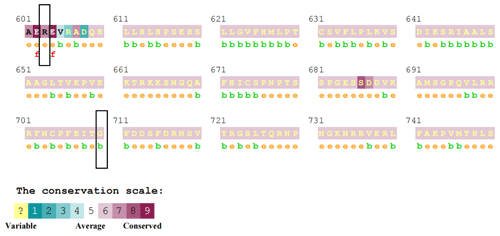
The prediction results of ConSurf online software showed that the R603S site score is 7, which belongs to the conservative site. The G710D site score is 6, which belongs to the neutral site (Fig. 3).
In this experiment, RT-qPCR was used to analyze the relative expression levels of the MLPH gene in Korean quail and Beijing white quail at different developmental stages. The results showed that the relative expression level of the MLPH gene in Beijing white quail was significantly higher than that in Korean quail, which is consistent with the research results that the relative expression level of the MLPH gene in white goat skin tissue was significantly higher than that in black goat and brown goat skin tissues (Fu, 2013). Lee et al. (2015) also showed that the relative expression level of the MLPH gene in Korean zebra cattle was significantly higher than that in brown cattle. The relative expression level of the MLPH gene was the highest on the eighth day of embryonic development in Beijing white quail, indicating that the gene was active at this time and affected the transportation and synthesis of melanin, thereby affecting the plumage color phenotype of quails. It was speculated that the occurrence of the white plumage phenotype of quails might be related to the high expression of the gene. Studies have shown that MLPH gene expression was detected in skin tissue of Zhedong white goose (Liu et al., 2016). This is also consistent with the results of the high expression of the MLPH gene in the skin tissue of Beijing white quail. The above studies have shown that the MLPH gene is related to the light color phenotype in animals, but its main expression and regulation mechanism need further study.
Table 5Prediction results of destructiveness of the MLPH gene nsSNPs.

PROVEAN: score is a deleterious mutation, and score > −2.5 is a neutral mutation. PolyPhen-2: score < 0.50 is benign; score ≥ 0.05 is destructible. SNAP2: score > 0 is influential; score < 0 variation is neutral. I-Mutant 3.0: score < −0.5 means reducing protein stability; −0.5 ≤ score ≤ 0.5 means variation is neutral; score > 0.5 means increasing protein stability.
Some studies have found that the mutation of the MLPH gene is related to the fading of animal coat color or plumage color. Posbergh et al. (2020) found that a mutation site (g.3451931C > A) of the MLPH gene in Jacob sheep led to the premature introduction of terminator codons into the protein, resulting in the loss of its function, thus causing the black coat of Jacob sheep to fade. The polymorphism analysis of the MLPH gene in rabbits with different coat colors showed that the mutation site of the MLPH gene (g.232766A < T) was related to the white phenotype of rabbits (Jia et al., 2021). Studies have shown that MLPH gene mutation can lead to melanosome transport defects, and the mutation site C.1909A > G was detected to cause plumage color fading in Anyi tile-like gray chicken, resulting in the “five-gray” phenotype (Xu et al., 2016). Vaez et al. (2008) determined that the mutation site of the MLPH gene (C.C103T) could lead to amino acid changes (R35W) by comparative analysis of chicken candidate gene sequences, and there was a significant correlation between the mutation site and the chicken plumage color dilution phenotype. Wang et al. (2022) found that the mutation of the MLPH gene was closely related to the lavender dilution phenotype of Baicheng You chicken. The results of this experiment also showed that the c.1807C > A mutation site of the quail MLPH gene was significantly correlated with its white plumage phenotype. The mutation sites of the MLPH gene found in the above study are not consistent with those in this study, which may be due to the different mutation sites of the MLPH gene between different species. The above studies also showed that MLPH gene mutation can cause animal coat color or plumage color fading. In addition to the MLPH gene, there are some other genes associated with avian plumage color. Noor et al. (2021) found that PMEL17 gene mutation was associated with the plumage color of Bangladeshi indigenous chicken, and the plumage color of indigenous chicken was associated with production performance. The MLPH gene was also studied in Japanese quail. Minvielle et al. (2009) found that a single mutation of the MLPH gene could lead to dilution of plumage color in Japanese quail. Bed'hom et al. (2012) also found that the complex mutation of the MLPH gene was related to the dilution of Japanese quail plumage color, which is consistent with the results of this experiment.
The frequency distributions of the three genotypes in Korean quail at two mutation sites (c.1807C > A and c.2129G > A) of the MLPH gene in this study were significantly different from those in Beijing white quail (P<0.01). Bioinformatic prediction showed that c.1807C > A was a neutral mutation and that c.2129G > A was a deleterious mutation. Conservative prediction of protein showed that the mutation sites of encoding proteins R603S and G710D caused by the two sites were a conservative site and neutral site, respectively, indicating that the two mutation sites might not cause changes in protein structure. However, association analysis showed that allele A at the c.1807C > A mutation site had an absolute advantage in the white quail population, suggesting that the occurrence of the white phenotype in the quail population might be related to the fact that allele A at this mutation site was a dominant gene in the population. Combined with the above studies, it can be inferred that MLPH gene mutation may play an important regulatory role in quail plumage color fading.
The relative expression level of the MLPH gene in Beijing white quail was significantly higher than that in Korean quail. The c.1807C > A mutation site in the quail MLPH gene was significantly correlated with its white plumage phenotype, and the c.2129G > A mutation site was significantly associated with its plumage color phenotype. SNP1 (c.1807C > A) was a neutral mutation, and SNP2 (c.2129G > A) was a deleterious mutation. The mutation sites of coding proteins R603S and G710D caused by SNP1 (c.1807C > A) and SNP2 (c.2129G > A) were highly conserved. The results can provide a reference for subsequent animal plumage color or coat color breeding.
The bioinformatics software packages used in this study can be used according to the URL provided in Table 2.
RNA-Seq data are from the NCBI database (accession number: PRJNA756792). The remaining data are available from the corresponding author upon request.
ZY, XZ, YP and YQ conceived and designed the experiments. ZY, QW, YH, YZ, SR and LH performed the experiments; ZY performed statistical analyses of experimental data. ZY prepared the draft of the manuscript. All authors critically revised the manuscript and approved the final version.
The contact author has declared that none of the authors has any competing interests.
The study protocol was approved by the Animal Care Committee of Henan University of Science and Technology.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors are thankful to all members of the 517 laboratory who provided expertise that assisted the study.
This research has been supported by the Henan Natural Science Foundation of China (grant no. 202300410153).
This paper was edited by Henry Reyer and reviewed by two anonymous referees.
Bauer, A., Kehl, A., Jagannathan, V., and Leeb, T.: A novel MLPH variant in dogs with coat colour dilution, Anim. Genet., 49, 94–97, https://doi.org/10.1111/age.12632, 2018.
Bed'hom, B., Vaez, M., Coville, J. L., Gourichon, D., Chastel, O., Follett, S., Burke, T., and Minvielle, F.: The lavender plumage colour in Japanese quail is associated with a complex mutation in the region of MLPH that is related to differences in growth, feed consumption and body temperature, BMC Genomics, 13, p. 442, https://doi.org/10.1186/1471-2164-13-442, 2012.
Doig, T. N., Hume, D. A., Theocharidis, T., Goodlad, J. R., Gregory, C. D., and Freeman, T. C.: Coexpression analysis of large cancer datasets provides insight into the cellular phenotypes of the tumour microenvironment, BMC Genomics, 14, p. 469, https://doi.org/10.1186/1471-2164-14-469, 2013.
Fu, X.: Study on differental expression of MLPH gene and the variations of the intron mutation among different colour Goat skin tissue, Master's thesis, Hebei Agricultural University, Baoding, 2013.
Galeb, H. A., Wilkinson, E. L., Stowell, A. F., Lin, H., Murphy, S. T., Martin-Hirsch, P. L., Mort, R. L., Taylor, A. M., and Hardy, J. G.: Melanins as sustainable resources for advanced biotechnological applications, Global Challenges, 5, 2000102, https://doi.org/10.1002/gch2.202000102, 2021.
Ishida, Y., David, V. A., Eizirik, E., Schäffer, A. A., Neelam, B. A., Roelke, M. E., Hannah, S. S., O'Brien, S. J., and Menotti-Raymond, M.: A homozygous single-base deletion in MLPH causes the dilute coat color phenotype in the domestic cat, Genomics, 88, 698–705, https://doi.org/10.1016/j.ygeno.2006.06.006, 2006.
Jia, X., Ding, P., Chen, S., Zhao, S., Wang, J., and Lai, S.: Analysis of MC1R, MITF, TYR, TYRP1, and MLPH genes polymorphism in four rabbit breeds with different coat colors, Animals, 11, p. 81, https://doi.org/10.3390/ani11010081, 2021.
Lee, H., Hee, P. J., and Gug, K. J.: Expression of coat color associated genes in Korean brindle cattle by microarray analysis, J. Anim. Reprod. Biotechnol., 30, 99–107, 2015.
Lee, J. A., Hwang, S. J., Hong, S. C., Myung, C. H., Lee, J. E., Park, J. I., and Hwang, J. S.: Identification of microRNA targeting mlph and affecting melanosome transport, Biomolecules, 9, p. 265, https://doi.org/10.3390/biom9070265, 2019.
Lehner, S., Gähle, M., Dierks, C., Stelter, R., Gerber, J., Brehm, R., and Distl, O.: Two-exon skipping within MLPH is associated with coat color dilution in rabbits, PloS One, 8, e84525, https://doi.org/10.1371/journal.pone.0084525, 2013.
Li, S., Wang, C., Yu, W., Zhao, S., and Gong, Y.: Identification of genes related to white and black plumage formation by RNA-Seq from white and black feather bulbs in ducks, PloS One, 7, e36592, https://doi.org/10.1371/journal.pone.0036592, 2012.
Li, Y. X., Zhang, X. H., Pang, Y. Z., Qi, Y. X., and Zhao, S. J.: Construction of MC1R and ASIP eukaryotic expression vector and its regulation of plumage color in Japanese quail (Coturnix japonica), J. Poult. Sci., 56, 84–90, https://doi.org/10.2141/jpsa.0180058, 2019.
Liu, J., Liu, Y., Wang, C., Yang, Y. Z., Wang, H. Y., Liu, A. F., and He, D. Q.: Molecular cloning, tissue expression and SNP identification of MLPH gene in goose, China Poultry, 38, 4–9, https://doi.org/10.16372/j.issn.1004-6364.2016.12.002, 2016.
Livak, K. J. and Schmittgen, T. D.: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods, 25, 402–408, https://doi.org/10.1006/meth.2001.1262, 2001.
Manakhov, A. D., Andreeva, T. V., Trapezov, O. V., Kolchanov, N. A., and Rogaev, E. I.: Genome analysis identifies the mutant genes for common industrial Silverblue and Hedlund white coat colours in American mink, Sci. Rep., 9, 4581, https://doi.org/10.1038/s41598-019-40918-7, 2019.
Mastrangelo, S., Cendron, F., Sottile, G., Niero, G., Portolano, B., Biscarini, F., and Cassandro, M.: Genome-wide Analyses Identifies Known and New Markers Responsible of Chicken Plumage Color, Animals, 10, p. 493, https://doi.org/10.3390/ani10030493, 2020.
Minvielle, F., Cecchi, T., Passamonti, P., Gourichon, D., and Renieri, C.: Plumage colour mutations and melanins in the feathers of the Japanese quail: a first comparison, Anim. Genet., 40, 971–974, https://doi.org/10.1111/j.1365-2052.2009.01929.x, 2009.
Moravčíková, N., Kasarda, R., Židek, R., Vostrý, L., Vostrá-Vydrová, H., Vašek, J., and Čílová, D.: Czechoslovakian wolfdog genomic divergence from its ancestors canis lupus, german shepherd dog, and different sheepdogs of european origin, Genes, 12, p. 832, https://doi.org/10.3390/genes12060832, 2021.
Myung, C. H., Lee, J. E., Jo, C. S., Park, J. I., and Hwang, J. S.: Regulation of Melanophilin (Mlph) gene expression by the glucocorticoid receptor (GR), Sci. Rep., 11, 16813, https://doi.org/10.1038/s41598-021-96276-w, 2021.
Nagy, S., Poczai, P., Cernák, I., Gorji, A. M., Hegedűs, G., and Taller, J.: PICcalc: an online program to calculate polymorphic information content for molecular genetic studies, Biochem. Genet., 50, 670–672, https://doi.org/10.1007/s10528-012-9509-1, 2012.
Nam, I. S., Oh, M. G., Nam, M. S., and Kim, W. S.: Specific mutations in the genes of MC1R and TYR have an important influence on the determination of pheomelanin pigmentation in Korean native chickens, J. Adv. Vet. Anim. Res., 8, 266–273, https://doi.org/10.5455/javar.2021.h511, 2021.
Noor, J., Khan, M. K. I., Momin, M. M., Das, A., Wright, D., Alvarez-Rodriguez, M., Rodriguez-Martinez, H.: Effect of PMEL17 plumage colour gene diversity on production performance of indigenous chicken variety of Bangladesh, Ind. J. Anim. Res., 55, 371–377, https://doi.org/10.18805/ijar.B-1285, 2021.
Posbergh, C. J., Staiger, E. A., and Huson, H. J.: A stop-gain mutation within MLPH is responsible for the lilac dilution observed in jacob sheep, Genes, 11, p. 618, https://doi.org/10.3390/genes11060618, 2020.
Raghunath, A., Sambarey, A., Sharma, N., Mahadevan, U., and Chandra, N.: A molecular systems approach to modelling human skin pigmentation: identifying underlying pathways and critical components, BMC Res. Notes, 8, p. 170, https://doi.org/10.1186/s13104-015-1128-6, 2015.
Vaez, M., Follett, S. A., Bed'hom, B., Gourichon, D., Tixier-Boichard, M., and Burke, T.: A single point-mutation within the melanophilin gene causes the lavender plumage colour dilution phenotype in the chicken, BMC Genetics, 9, p. 7, https://doi.org/10.1186/1471-2156-9-7, 2008.
Wakabayashi, T., Takei, A., Okada, N., Shinohara, M., Takahashi, M., Nagashima, S., Okada, K., Ebihara, K., and Ishibashi, S.: A novel SOX10 nonsense mutation in a patient with Kallmann syndrome and Waardenburg syndrome, Endocrinology, diabetes and metabolism case reports, 20-0145, https://doi.org/10.1530/edm-20-0145, 2021.
Wang, H., Wen, J., Li, H., Zhu, T., Zhao, X., Zhang, J., Zhang, X., Tang, C., Qu, L., and Gemingguli, M.: Candidate pigmentation genes related to feather color variation in an indigenous chicken breed revealed by whole genome data, Front. Genet., 13, 985228, https://doi.org/10.3389/fgene.2022.985228,2022.
Xi, Y., Wang, L., Liu, H., Ma, S., Li, Y., Li, L., Wang, J., Chunchun, H., Bai, L., Mustafa, A., and He, H.: A 14-bp insertion in endothelin receptor B-like (EDNRB2) is associated with white plumage in Chinese geese, BMC Genomics, 21, p. 162, https://doi.org/10.1186/s12864-020-6562-8, 2020.
Xu, J. G., Xie, M. G., Zou, S. Y., Liu, X. F., Li, X. H., Xie, J. F., and Zhang, X. Q.: Interactions of allele E of the MC1R gene with FM and mutations in the MLPH gene cause the five-gray phenotype in the Anyi tile-like gray chicken, Genet. Mol. Res., 15, 15027633, https://doi.org/10.4238/gmr.15027633, 2016.
Xu, Y., Zhang, X. H., and Pang, Y. Z.: Association of Tyrosinase (TYR) and Tyrosinase-related Protein 1 (TYRP1) with melanic plumage color in Korean quails (Coturnix coturnix), Asian. Austral. J. Anim., 26, 1518–1522, https://doi.org/10.5713/ajas.2013.13162, 2013.
Yuan, Z. W., Zhang, X. H., Pang, Y. Z., Qi, Y. X., Wang, Q. K., Ren, S. W., Hu, Y. Q., Zhao, Y. W., Wang, T., and Huo, L. K.: Screening of stably expressed internal reference genes for quantitative real-time PCR analysis in quail, Biol. Bull., 49, 418–427, https://doi.org/10.1134/S1062359022050223, 2022.
Zhang, T., Sun, Y., Zheng, T., Wang, R., Jia, D., and Zhang, W.: MLPH accelerates the epithelial-mesenchymal transition in prostate cancer, OncoTargets Ther., 13, 701–708, https://doi.org/10.2147/ott.S225023, 2020.