the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Status quo of genetic improvement in local goats: a review
Glafiro Torres-Hernández
Jorge Alonso Maldonado-Jáquez
Lorenzo Danilo Granados-Rivera
Homero Salinas-González
Gabriela Castillo-Hernández
This review aims to summarize and synthesize the fragmented information available on the genetic improvement of local goats (criollo, indigenous, native) on the American and other continents, where populations with these goats have an important role in food security and the economy of rural communities, as well as in conservation of biodiversity and productivity improvement. Topics such as the current state of goat production globally, conservation programs, resistance to parasites and diseases, use of phenotypical characteristics and genomic information, and molecular markers for genetic improvement are addressed. The main challenges, opportunities, and limitations described in recent literature concerning local goats in the immediate future are discussed.
- Article
(198 KB) - Full-text XML
- BibTeX
- EndNote
Goats have been a food source for humans for many years, since their expansion throughout the world (Sevane et al., 2018). Worldwide, there are around 1094 million goats; most of them (94 %) are in countries of Asia and Africa, followed by the Americas (3.4 %) and Europe (1.4 %) (FAOSTAT, 2019). In China, there are around 58 domestic local breeds, distributed in different environments (Du et al., 2011). India has 23 well-recognized goat breeds (Mandal et al., 2014). In Africa, most of the goats (63 % of the population) are non-improved native breeds (Visser and van Marle-Köster, 2017), and in the Americas, there are around 28 recognized local breeds (Boettcher et al., 2014). In addition, the majority of these populations are utilized for self-consumption of products such as milk, meat, and skin or fibers, and they are found in herds that belong to low-income producers (Dubeuf and Boyazoglu, 2009).
On the other hand, the available information about genetic improvement (GI) of local goats (Montaldo et al., 2010), also known as criollo, indigenous, or native, in the Americas and other regions of the world is limited; however, the available results indicate that local breeds have a high potential for improvement (Argyriadou et al., 2020). In addition, the variation between and within these breeds is highly valuable, since great genetic variability is required for the populations to adapt and face the environmental changes that they are subjected to (Ojo et al., 2015). In time, GI has been limited to the “commercial” breeds, although their low participation in the animal registry and improvement plans has resulted in slow progress and an accelerated loss of genetic variability (Visser and van Marle-Köster, 2017).
In this sense, the incipient generation of information about populations of local goats is due, in principle, to the fact that there is the notion that they have a lower productive and economic performance, which decreases interest among producers and places a specific breed or genotype at risk of extinction (Biscarini et al., 2015). Therefore, the greatest challenge of GI in local breeds is the adaptation of selection schemes and classic improvement in small populations and production systems characterized by poor organization from producers (Gandini et al., 2017). In addition, it is necessary to include the ideology of small-scale producers in the objectives and selection criteria. For example, Gunia et al. (2010) mention that the conformation and growth of males are among of the most desirable traits for producers in the Caribbean (77 % of the interview respondents), followed by maternal behavior, reproduction, and milk production. They conclude that GI programs must consider the use of local populations as maternal lines.
1.1 Use of exotic germplasm and its impact on local populations
In countries like Iraq, Syria, Morocco, and Tunisia, efforts have been directed to design GI programs, which have consisted of the distribution of pure breed bucks (Saanen, Nubian, Toggenburg, Alpine, etc.). So, this has not had the foreseen success due to the low adaptation of bucks to the environment. In addition, there is limited participation of producers in the design of GI plans (Iñiguez-Rojas et al., 2013). Consequently, farmers are not motivated to invest in these programs, resulting in intermittent implementation and low success (Argyriadou et al., 2020). This situation is similar in Latin American countries such as Mexico (Montaldo et al., 2010; Alejandre-Ortiz et al., 2016), Ecuador (Gómez-Carpio et al., 2016), Peru (Gómez-Urviola et al., 2016), and Bolivia (Stemmer and Valle Zárate, 2016).
The goats that migrated to the “new world” (the West) have European, African, and Central Asian origins, from which the local goats found in the Americas are derived and represent a reservoir of genetic diversity to be used in breeding programs, selection, and conservation (Paim et al., 2019). Genetic improvement has been done in Brazil by introducing goat populations from Portugal and Spain and more recently from Asia, Africa, and Europe (Braga-Lôbo et al., 2010). This caused these goats to go through intense adaptation processes to overcome severe climate conditions in this country, particularly in the northeast of Brazil, thus originating a particular goat type that is clearly separate from the other local breeds of the rest of the American continent, with a marked influence from Cape Verde goats (Sevane et al., 2018).
Deza (2007) proposed a genetic improvement plan in Argentina for local goats in “agroecologically restrictive” environments, stemming from the characteristics attributed to the local goat. Among these characteristics, the following stand out: animals that are polymorphic, polychromic, rustic, and adapted to extreme environments, which have not been sufficiently described and therefore have still-unknown productive potential, with high productive variability between and within the herds. For this reason, most of the efforts to conduct GI in these populations continue to be done traditionally (Ahmed et al., 2020).
1.2 Community-based breeding programs
In countries such as Bangladesh, community-based breeding programs (CBBPs) have been established, whereby, contrary to what was mentioned before, producers participate with the common interest of conserving and improving their genetic resources in low-income production systems (Bhuiyan et al., 2017). Liberia also incorporated a pilot CBBP in three countries with the participation of goat farmers (Karnuah et al., 2018a). In Ethiopia, the implementation of this GI plan increased producers' income by 20 % by improving the size of the litter. In addition, meat consumption at the farm level increased, so a CBBP is considered a feasible plan with measurable genetic gains in performance characteristics and the impact on the standard of living of producers (Haile et al., 2020).
In Turkey, positive effects were observed beyond improving the animals, since the living standard of producers and some market situations improved; however, a critical success factor for these programs is the monitoring and continuous training of the producer (Saatci et al., 2017). Likewise, Mueller (2017) evaluates the characteristics of some CBBP cases for the production of dairy goats in Mexico, Cashmere goats in Iran, Mohair goats in Argentina, and dairy goats in Kenya. The study concludes that planning and participation by local institutions are of utmost importance for support in the organization, financing, and technical support of the participating producers. Since one of the main problems faced by this strategy is sustainability in time, GI efforts for local goats must be conducted from an integral perspective (Wurzinger et al., 2021).
For example, in Malawi and Uganda, the use of CBBPs increased growth performance and survival of offspring; however, to achieve adoption of this model, it is recommended to establish producer cooperatives and reform regional and supportive policies (Kaumbata et al., 2021). Also, it must inevitably correlate the existing genotypes with the environment without underestimating its potential for improvement and emphasizing the need to make more significant conservation efforts, such as what has been proposed in Kenya (Rewe et al., 2002). Given the variability of CBBP success in Ethiopia, valuable recommendations have been generated that could improve the impact of these programs. The actions to be implemented are reproductive improvements such as artificial insemination and synchronization of oestrus, which will allow knowing the pedigree of the animals, removal of the effect of the birth season, and acceleration of the breeding process with the best identified individuals (Weldemariam and Mezgebe, 2021).
1.3 Conservation programs
Knowledge of the genetic structure of organisms, genetic populations, and phylogenetic studies is essential to provide opinions about effective conservation and reintroduction plans, mainly when the target population is at risk of extinction (Ariyaranthe et al., 2016). For the specific case of local goats, these have high significance due to their unique adaptation characteristics, with a particular relevance because of their low maintenance, which makes them a vital ally to face and combat the effects of climate change (Monau et al., 2020). In this sense, some characterization efforts in Sri Lanka, Slovenia, and Greece have found that local goat populations have a distinct genetic identity and are heterogeneous. The contemporary structure of the population is highly influenced by anthropogenic actions, making it necessary to establish immediate activities for their conservation (Ariyaranthe et al., 2016; Michailidou et al., 2019; Pogorevc et al., 2021). However, the lack of emphasis on production based on technology and appropriate characterization of breeds remains a limiting factor for the sustainable use of these goats (Monau et al., 2020).
Liu et al. (2019) conducted a study to define conservation priorities of 26 local breeds in China to establish protection programs according to the importance within and between breeds given by genetic diversity, and they found that the Inner Mongolia Cashmere, Jining Gray, and Liaoning Cashmere goats were the ones that contributed most to heterozygosity and total diversity. However, for the case of Daiyun and Shannan Blanca goats, their conservation should be prioritized based on the population's effective size.
Due to the low productive indices of local goats in Brazil, “exotic” breeds were introduced, which, even though they are more productive, do not have adaptation characteristics (resistance to diseases and parasites) that local goats have. Despite this fact, exotic breeds have substituted local breed (Canindé, Gurguéia, Moxotó, Marota, and Repartida), placing the latter in danger of extinction. To avoid the loss of this genetic resource, the Program for Conservation of Animal Genetic Resources was created through “conservation nuclei” in habitats where the goats are subject to natural selection (in situ) and also from storage of semen and embryos (ex situ) (Mariante et al., 1999).
In Cuba, an exploration of 48 herds that were thought to be “local” was performed, finding that only one of them was indeed local. Therefore, a research–action process was implemented for the conservation of this zoogenetic resource, with the active participation of farmers facing an adverse environment with social complexities (Chacón-Marcheco et al., 2016). Similarly, this effort has been carried out in countries like Mexico (Salinas-González et al., 2011, 2013), where the conservation process resulted in a fundamental scenario for the development of several studies, which have contributed valuable information related to genetic characteristics. With this learning, peasant systems acquired new development opportunities, but they conserved the socioeconomic rationalities for breeding, whose capacity to “change and conserve at the same time” was called “socioecological resilience” (Chacón-Marcheco et al., 2016). Within this context of innovation for conservation, there were community livestock fairs held as a tool to revitalize the participative management of zoogenetic resources. This strategy eases the flow of specimens through purchase–sale practices or solidary practices of exchange and loan.
Another conservation program for local goats that has entailed good planning, organization, and monitoring has taken place in the French Antilles (Naves et al., 2016) and to a lesser degree in Ecuador (Gómez-Carpio et al., 2016). In Mexico, without official programs, the conservation of local goats in some regions has been found to be a necessity by the producers themselves, who have confirmed the advantages of local genotypes compared to the improved breeds that have been introduced. Some examples are the Tarahumara goat in Sierra de Chihuahua (Alejandre-Ortiz et al., 2016), the White Celtiberian goat in Zacatecas (Reveles-Torres et al., 2008; Sánchez-Gutiérrez et al., 2021), the local goats in Comarca Lagunera (Escareño-Sánchez et al., 2011; Maldonado-Jáquez et al., 2018), the Black goats in Querétaro (Andrade-Montemayor, 2017), and the Pastoreña goat from the Mixteca regions in Puebla and Oaxaca (García-Bonilla et al., 2018; Villarreal-Arellano et al., 2020).
Likewise, in France, the interest in the conservation of biodiversity of domestic animals, including goats, has been “rekindled”, since a fast decrease or even disappearance of certain breeds has been observed. Therefore, the choice of “returning” to grazing as an option to conserve these local zoogenetic resources is being studied, since historically extensive livestock production has been linked to local aspects, traditional breeds, and therefore direct conservation (Bertaglia et al., 2005).
2.1 Resistance to gastrointestinal nematodes
The most significant efforts for research on this topic have been made with local goats from the French Antilles (de la Chevrotière et al., 2011; Bambou et al., 2013; Mandonnet et al., 2014). However, Brazil maintains an improvement program between breeds that also includes resistance to nematodes (Costa et al., 2000).
Infections due to gastrointestinal nematodes (GINs) in small ruminants represent the main limitation of production in grazing systems in the tropics because they cause economic losses when decreasing the production (Miller and Horohov, 2006; Preston et al., 2014). In that regard, anthelmintic products to control GINs had success for many years; however, resistance to these products has spread throughout the world.
Concerning this, Costa et al. (2000) studied the variability between and within goat breeds by measuring the number of eggs per gram of feces (EPG), packed cell volume (PCV), and hemoglobin (HB) in local kids of the breeds Canindé and Bhuj, as well as in Nubia, all exposed to Haemonchus contortus. The results indicated a negative correlation between egg counts and blood values and suggest differences between breeds in PCV and HB; this, in turn, is related to resistance to infection by H. contortus or the effects.
Another alternative to counteract the problem of parasitosis from gastrointestinal nematodes is dietary supplementation. In this regard, Torres-Acosta et al. (2006) studied the effect of dietary supplementation in local kids that graze on native vegetation during the humid season in Yucatán, Mexico, on resilience to infections by GINs. The results showed that dietary supplementation improved the kids' resilience to GIN infections, which, in addition, is an economically viable technology.
Creole goats from the Guadeloupe islands in the Caribbean are one of the most studied breeds regarding resistance to infections by GINs. This genetic resistance has been studied since 1995 because they are very susceptible to these infections. In this regard, Mandonnet et al. (2001) studied the genetic variation for resistance to infection from nematodes to introduce this variable into genetic improvement plans. The researchers found a heritability value (h2) for EPG of 0.37±0.06 at weaning and between 0.14±0.05 and 0.33±0.06 at 4 and 10 months during fattening. The h2 for PCV varied from 0.10 to 0.33 with maternal and direct h2 values at weaning for EPG of 0.26 and 0.20, respectively; genetic correlations between PCV and body weight decreased from 0.47 to 0.10 between weaning and 10 months of age, when the kids are infected by Haemonchus contortus and Trichostrongylus colubriformis, showing that the GI for resistance to GINs is viable.
In their review, Mandonnet et al. (2014) addressed the appropriateness and viability of performing the selection of local goats based on their resistance to GINs and developed three strategies to face this problem: (a) reducing the host's contact with the infectious larvae through a reduced load in grazing, (b) extending the efficiency of molecules from synthetic anthelmintic molecules by implementing directed selective treatments or the use of phytotherapeutic drugs, and (c) increasing the ability of the host to tolerate the negative effects of the worms (resilience) and eventually responding to the parasites (resistance) from genetic selection.
The problem of infections from GINs has been studied at the molecular genetic level. In this regard, a large number of loci for quantitative characteristics have been detected (QTLs) associated with resistance to GINs in small ruminants in more than 20 chromosomal regions (Dominik, 2005; Bishop and Morris, 2007). The first scan of the genome for resistance to GINs in goats was carried out in local goats from Guadeloupe (de la Chevrotière et al., 2011) and identified 13 QTLs for resistance, resilience, and immunity criteria. Later, after performing a quality control analysis of SNPs (single-nucleotide polymorphisms), they identified 46 643 markers for studies by GWASs (genome-wide association studies) (Silva et al., 2018).
Likewise, Aguilar-Caballero et al. (2008), in their review, highlighted the existence of resilience and resistance as natural defense mechanisms of local goats against GINs. In addition, they discussed the situation of gastrointestinal parasitosis in goats, the control measures applied, their current situation, and the alternative methods for GINs control-tested in goats that can be transferred to the field (Aguilar-Caballero et al., 2011).
2.2 Resistance to diseases
Disease resistance is a topic in which the component of genetic resistance has been studied as part of control mechanisms, not only because of its impact on productivity but also because of the zoonotic and economic implications that it represents (Buhari et al., 2020; Palomares-Reséndiz et al., 2021). In this sense, this genetic resistance is considered an alternative for the control of diseases and the use of antimicrobials; it includes both immune and non-immune mechanisms and is defined as the inherent capacity of an animal not previously exposed to pathogens. It has been found that natural resistance is inherited and passed from parents to offspring (Adams and Templeton, 1998). This paper will consider some studies that have evaluated the genetic resistance of local goats to various diseases.
Brucellosis is a zoonotic disease of public importance in countries such as Mexico, Nigeria, and Algeria, which is caused by the bacterium Brucella melitensis. Regarding this disease, transmission patterns and dynamics have been studied, as have some induced and natural resistance mechanisms. To date, it has been found that genetic resistance to intracellular pathogens is linked to a genomic region of the SLC11A1 gene, which is considered a candidate for resistance to this disease (Sahraoui et al., 2020; Palomares-Reséndiz et al., 2021).
On the other hand, hydrocarditis or aqueous heart (“cowdriosis” or “heartwater”) is an acute infectious disease that is non-contagious and fatal. It is produced by rickettsiae in ruminants, caused by Ehrlichia ruminantium, and transmitted by Amblyomma ticks. So, it occurs in nearly all the countries of Africa and nearby islands, as well as the Caribbean. The disease can cause high mortality in susceptible domestic ruminants (up to 90 %); goats and sheep are more vulnerable than cattle and European breeds more than African ones (Teklu et al., 2017; Dinkisa, 2018). In this regard, Bensaid et al. (1993) suspected resistance to this disease in Creole goats from Guadeloupe, and therefore Bambou et al. (2010) performed a study to confirm this resistance and susceptibility in local goats to a standardized sub-lethal infection of E. ruminantum, inducing 70 % mortality. They measured the intensity of the disease using clinical reaction indices (incubation period, fever intensity, nervous signs, and death), and differences were found between the mortality rate after infection. This was the first study wherein genetic variability of this disease was found, and there is an attempt to link this variability physically with areas of the genome. Likewise, Matheron et al. (1987) concluded that resistance seems to be under genetic control since a herd studied for 10 years and exposed to the disease reached a resistance rate of 78 %.
Even more, the goat arthritis encephalitis (GAE) is a chronic disease eased by a retrovirus of the Lentivirus genus. There are no vaccines or effective treatments. Therefore, the hypothesis has been suggested that through genetic selection, there could be a feasible alternative for the control of this disease, as reported by Schultz et al. (2020), who estimated heritability values of 0.077 (with ranges of 0.026 to 0.128) for resistance to GAE in Alpine and Saanen goats.
Kim et al. (2019) found candidate genes for resistance to Salmonella (LBP and BPI) and heart disease (TTN and ITGB6) in Korean, Iranian, and Moroccan goats, which is of maximum importance to direct future efforts to conserve species biodiversity and contribute to genomic improvement programs. For their part, Meydan et al. (2017), identified resistant genotypes in variants of the gene that encodes the prion protein (K222), observing a decrease in “tremors” caused by Scrapie's disease. This resistance has been observed in local goats from Italy (Migliore et al., 2015).
Phenotypical characterization is the first phase to document the qualitative and quantitative characteristics of local goats. In this regard, a large variety of colors, types of beards, horns, ears, and hair, among others, has been reported (Table 1). Most of the variations observed in phenotypical characterization studies are due to environmental factors, topography, and vegetation, which give rise to different ecotypes (Monau et al., 2020). In addition, it fosters differences between and within breeds (Table 2). For example, the withers height of Bolivian local goats (51–58 cm) is lower when compared to that of Sabanero local goats from Colombia (75–80 cm). The great difference in body length between local goats from Arque, Bolivia (47 cm), and Amatepec, Mexico (104 cm), is also worth noting, representing a difference higher than 100 %. Even among the same local goats, there are differences for these same characteristics (Stemmer and Valle-Zárate, 2016).
Table 1Phaneroptic characteristics in local goats from various countries of the world.
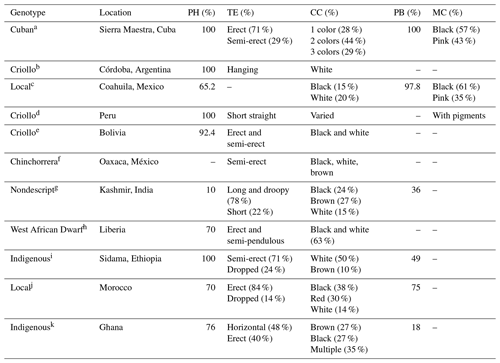
PH: presence of horns; TE: type of ears; CC: coat color; PB: presence of beard; MC: mucosa color; a Chacón et al. (2011), b Deza et al. (2007), c Moyao-Ariza et al. (2022), d Gómez-Urviola et al. (2016), e Stemmer and Valle-Zárate (2016); f Ortiz-Morales et al. (2021), g Ali Rather et al. (2020); h Karnuah et al. (2018b), i Hankamo et al. (2020), j El Moutchou et al. (2017), k Hagan et al. (2012).
In this sense, the external characteristics of local goats are an essential selection criterion that must be considered, since they are the first impression of the animal. It is a critical point to ensure the dissemination of the genetic material of those valuable animals and to guarantee appropriate genetic progress (Labatut et al., 2013). In this regard, producers from Patagonia, Argentina, and Oromia (Ethiopia) use several morphometric and phaneroptic criteria to select their replacements, highlighting the size, animal conformation, type of hair, and coat color (Lanari et al., 2005; Bedada et al., 2019).
Table 2Morphometric characteristics (mean ± SD, cm) of local goats in diverse regions of the world.
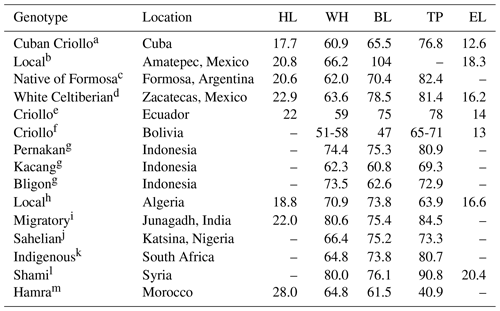
HL: head length; WH: withers height; BL: body length; TP: thoracic perimeter; EL: ear length; a Chacón et al. (2011), b Dorantes-Coronado et al. (2015), c Prieto et al. (2006), d Sánchez-Gutiérrez et al. (2018), e Gómez-Carpio et al. (2016), f Stemmer and Valle-Zárate (2016), g Alawiansyah et al. (2020), h Benyoub et al. (2018), i Patbandha et al. (2018), j Rotimi et al. (2020), k Tyasi et al. (2020); l Hassen et al. (2016), m Hilal et al. (2016).
Valencia-Posadas et al. (2017) evaluated the phenotypical relations between conformation and milk production traits and reported that goats with better body conformation were not the best to produce milk, which can be directly related to the productive aptitude of the genotype in question and should therefore be considered at the time of implementing GI schemes. Because of this, a new methodology has been developed that combines the use of phenotypical distribution models to predict the behavior of the livestock, and it has been concluded that it might be a highly valuable tool to evaluate and conserve the breeds that adjust better to specific environments (Lozano-Jaramillo et al., 2019).
Undoubtedly, data collection in productive phenotypical characteristics from producers, particularly under field conditions, represents devoting time, effort, and concentration, which in many cases is not enough to have a reliable database for their later use in GI programs. In this sense, phenomics, understood as the application of technologies that allow the collection of phenotypes easily, economically, and in large volumes, represents a great opportunity to achieve considerable improvements (Mrode et al., 2020), since, with the widespread growth of communication technologies, GI programs for local populations could be revolutionized in developing countries. Some examples include the use of sensors in females to detect patterns of reproductive behavior, the prediction of body weight in animals for meat production using a measuring tape and measurements of the thoracic perimeter, and the use of mobile devices that help to evaluate milk production and weight gain, among others (Mrode et al., 2020).
High-density genomic tools have been used in the last decade to study and characterize livestock's genetic diversity and population structure. However, many individual phenotypes and genotypes are required to obtain accurate genetic values, which in the case of local populations with low numbers of individuals can only expect low genetic gains (Biscarini et al., 2015). A large variety of techniques has been developed to help solve this problem in order to evaluate variability and diversity at the DNA level (Table 3). Nowadays, techniques are available, such as random amplified polymorphism DNA (RAPD), amplified fragment length polymorphism (AFLP), restriction fragment length polymorphism (RFLP), simple sequence repeat (SSR), and short tandem repeats (STRs), among others. According to the literature consulted, the most frequently used techniques to estimate the genetic diversity between populations of sheep, goats, bovines, buffaloes, camels, and horses are RAPD (Al-Barzinji and Hamad, 2017) and microsatellite markers or STRs (Arangurén-Méndez et al., 2005).
On the other hand, genome-wide association studies (GWASs) help to explore the relationship between genetic and phenotypic variation in terms of genomic markers, identifying variants that can be part, or not, of known genes. Stemming from this, molecular research has opened new possibilities for the genetic characterization, conservation, and utilization of the only genetic resource that local goats have (Visser and van Marle-Köster, 2017; Wittenburg et al., 2020). However, the use of these tools has not been implemented for goats on a broad scale. In that regard, in Greece, studies of this type in native goats are limited. Only microsatellite markers or SNPs have been used to characterize genetic distances between breeds based on polymorphisms of blood proteins, finding significant genetic variability and potential for improvement in productive and aptitude traits (Argyriadou et al., 2020). In Italy, studies with SNPs have been carried out to characterize the genetic diversity of native dairy goats such as Argentata dell'Etna, Derivata di Siria, Girgentana, and Messinese goats (Di Gerlando et al., 2020). Likewise, Ceccobelly et al. (2020) mention that molecular tools are of great help to discriminate breeds adequately at a low cost. In addition, they must be considered to trace goat products and distinguish their origin.
In India, Jayashree et al. (2019) conducted a study to identify the diversity of Karnakata goats through 23 microsatellite markers and found a heterogeneous local population, which is highly valuable because of its adaptation characteristics. Likewise, in Sri Lanka, many localized native goats cover most of the country's goat population and are at high risk of extinction from intensive crossing with exotic breeds. This situation positions them as an invaluable genetic resource from their high environmental adaptation and resistance to diseases. Therefore, SNPs have been used to identify regions of the genome that are correlated with the productivity, resistance, and fertility of goats (Ariyaranthe et al., 2017).
Based on the preliminary information, the use of these genomic and molecular tools has become of utmost importance for the improvement and conservation of native breeds in general, since they allow combining pedigree, phenotypes, and genotypes in a simple evaluation without the need for post-analysis processes (Lourenco et al., 2020).
In their review, Mrode et al. (2018) indicated that CBGI is a reasonable frame of reference; innovative genomic selection will be required in fragile growth systems wherein adaptation is an important characteristic, and it will be essential to identify regions of the genome related to aspects of adaptability with the aim of maximizing the diversity of animals. In addition, the same authors (Mrode et al., 2018) point out that an adequate cost–benefit analysis ought to be part of any strategy to implement genomic selection in these production systems.
An example of the use of these techniques is reported by Sevane et al. (2018), since they generated a perspective of the diversity of goats in the Americas using genomic tools, by using information from Iberian, African, and local breeds. For this case, they obtained important signatures of Iberian breeds, particularly Cuban Criollo, but with a significant contribution from African breeds that have given rise to many local breeds. With this information, there are advances in revealing the evolutionary history of local goats and defining conservation priorities to maintain the diversity that they have inherited.
Table 3Genomic–molecular studies carried out with local goats in various regions of the world.
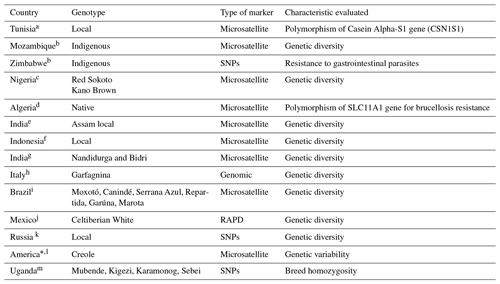
a Jemmali et al. (2012), b Monau et al. (2020), c Ojo et al. (2015), d Sahraoui et al. (2020), e Zaman et al. (2014); f Zein et al. (2012), g Tantia et al. (2018), h Dadousis et al. (2021), i Menezes et al. (2020), j Reveles-Torres et al. (2008), k Deniskova et al. (2021), l Ginja et al. (2017), m Onzima et al. (2018). * Sample of 910 animals from 10 countries.
5.1 Challenges
During the last century, erosion of goat genetic resources has been observed as a result of a massive replacement of “low-productivity” local breeds by other “highly productive” specialized breeds (Di Gerlando et al., 2020). However, the statement that specialized breeds are more productive is not entirely correct, since there is evidence suggesting that crossbreeding with pure breeds does not necessarily improve the productive aptitude of local genotypes under the management conditions in which these genotypes are traditionally developed. This way, the overall result is lower production than expected from the interaction of an unfavorable genotype with the environment (Lozano-Jaramillo et al., 2019). Evidence of this fact was given by Gaddour et al. (2010), who found that crosses of Damasco with Alpine and Murciano–Granadina goats improved milk production and kid growth, but negative effects in reproductive traits were also detected.
Due to the above, producers of local goats face the challenge of maintaining or increasing the size of their populations and thus avoiding extinction. Therefore, strategies should be sought that help apply all available tools to improve the productivity and profitability of these farms, including a wide range of GI technologies like genomic profiles, targeted genetic improvement schemes, and different reproductive, marketing, and traceability tools (Biscarini et al., 2015; Michailidou et al., 2019) that could help small-scale producers to survive. On the other hand, a critical challenge faced by producers is the availability of technical and scientific information, since, in general, information on new technological advances is available mostly among the scientific community; for producers, this information is massively disseminated because in some parts of the world, access to this type of information is highly restricted (Argyriadou et al., 2020) or the cost to access it is extremely high, which seriously affects decision-making in public policies and at the farm level. The abovementioned issue is not exclusive to rural areas, but this lack of information is also present in urban and metropolitan areas; therefore, the challenge is to generate strategies whereby new information on technologies and/or findings in any aspect of the production chain reaches producers and decision makers so that they can be applied. In addition, another of the great challenges observed is to seek strategies that help sustainability and mitigate the effects of climate change to guarantee the supply of food of animal origin to a growing population (Wattiaux, 2019).
5.2 Limitations
A severe limitation in GI programs for these populations is the lack of continuity between research and practical implementation, which is aggravated by the deficiency of resources to monitor these programs due in principle to the fact that government research and development programs have only considered productivity, without considering aspects of population adaptation. In this sense, some research objectives are altered based on scientific trends, resulting in intermittent studies without future efforts (Argyriadou et al., 2020). In that regard, Djemali (2000) points out that in countries such as Tunisia, those who have invested for decades in GI programs for local goats to be successful have found that governments hardly support animal registry and selection costs for prolonged periods. In addition, there is a scant connection between state entities that implement GI programs and researchers who are the ones with the “know-how” about what should be used. Furthermore, involvement from producers is not observed, leading to constant changes in the objective's selection, thus limiting the genetic progress. An example of the failure of these GI projects, wherein the producer is not considered, happened with goat producers in Morocco and Senegal (Kosgey et al., 2006). In other words, there is a will to improve the production schemes but a lack of real organization of all the actors involved. Concerning this, Giovannini (2011) suggested that the greatest limitations for the transference of GI plans are inadequate policies, precarious economic development, problems inherent to production systems, precarious institutional development, and precarious and unsustainable financing.
5.3 Opportunities
One of the great opportunities presented by local goats is exceptional genetic diversity and variability, which is essential to adapt production systems to future challenges and represents a critical point to face climate change and its effects (Paim et al., 2019).
Advances have been found for reproductive traits. Since the estimators of heritability for these traits are low, huge amounts of data are required for the GI process to be effective, which is complicated in local populations (Atoui et al., 2018). However, researchers can develop strategies to improve reproduction in local goat genotypes, given the advancement in genomic and molecular tools (Dagong et al., 2020). This could allow identifying genomic–genetic variants with relative ease, which would make it possible to apply scanning techniques of the genome, association studies in the genome, and genomic selection to increase prolificacy (Gomes de Lima et al., 2020). In addition, high genetic variability and a low level of inbreeding in native breeds have been found, indicating that there are no negative signs for the sustainable use of goats in the future (Karsli et al., 2020).
A wide range of possibilities has been explored for GI in populations of local goats. In that regard, Atoui et al. (2020) pointed out that some management practices seem to have an impact on estimating genetic components for growth in local kids; that is, the selection at critical ages that defines changes in the growth components seems to be more important when using frequent weighing. As pointed out by Torres-Hernández et al. (2020), this should be considered within GI programs for an accurate estimation of milk production through lactation. Although all the strategies contribute to knowledge, the main limitations for improving local goats in many parts of the world are fundamental issues such as diseases and parasites, poor nutrition (quality and quantity), genetic potential without quantifying, inadequate infrastructure and access to markets, minimal institutional and services support, and poor access and underutilization of knowledge, information, and technologies (Nwogwugwu et al., 2018).
Finally, countries such as Sudan and Italy have clearly defined the production objectives for their native genotypes and believe that the use of genomic–genetic technologies should be made widespread for the characterization of genotypes or breeds. It is thought that these would save considerable time and resources (El Hag et al., 2020) although emphasizing that special attention must be paid during the selection process to not altering the adaptation of goats to local conditions, specifically those characteristics that are of interest for conservation (Gandini et al., 2017). Therefore, an urgent need has been generated to consider different measures to address the limitations identified so far in terms of the GI of local goats to exploit the potential genetic diversity and benefit of these animals (Nwogwugwu et al., 2018).
Significant advances have been observed in recent years in the conservation and genetic improvement of local goats globally, primarily due to the last-generation molecular tools. However, it is still perceived that efforts are isolated and intermittent. They are still not enough to conserve and recover these populations, despite them being an invaluable reservoir of genetic material resistant to diseases and parasites that is adapted to produce and reproduce under extreme environmental conditions.
These characteristics make local goats one of the few viable and sustainable options of low economic, nutritional, and environmental requirements. They can help to recover both food self-sufficiency, with products of high biological value, and income in developing countries with extreme poverty facing the adverse effects of climate change.
Some other genetic improvement and conservation strategies for these populations should focus on the characterization of the productive system in an integral manner and where phenotypic and genotypic characterization should be considered part of the evaluation of the various mechanisms of adaptation to the environment that these goats have developed since this information can contribute to the development of programs for better comprehensive genetic improvement schemes.
Because of this, it is urgent to redouble efforts in research, support, and accompaniment in all the links of the productive chain that seek practical solutions to the significant problems presented by the production of one of the noblest animal species that has been vital for the development of humanity.
The data are available upon reasonable request from the corresponding author.
GTH was responsible for conceptualization, supervision, and writing (review and editing). JAMJ was responsible for conceptualization and writing (original draft preparation). LDGR was responsible for conceptualization and writing (review and editing). HSG was responsible for validation and writing (review and editing). GCH was responsible for original draft preparation and validation.
The contact author has declared that neither they nor their co-authors have any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors thank INIFAP and Colegio de Postgraduados for financial support.
This paper was edited by Steffen Maak and reviewed by three anonymous referees.
Adams, L. G. and Templeton, J. W.: Genetic resistance to bacterial diseases of animals, Rev. Sci. Tech., 17, 200–219, https://doi.org/10.20506/rst.17.1.1085, 1998.
Aguilar-Caballero, A. J., Torres-Acosta, J. F. J., Cámara-Sarmiento, R., Hoste, H., and Sandoval-Castro, C. A.: Inmunidad contra los nematodos gastrointestinales: la historia caprina, Trop. Subtrop. Agroec., 9, 73–82, 2008.
Aguilar-Caballero, A. J., Cámara-Sarmiento, R., Torres-Acosta, J. F. J., and Sandoval-Castro, C. A.: El control de los nematodos gastrointestinales en caprinos: ?`dónde estamos?, Bioagrociencias, 4, 1–16, 2011.
Ahmed, R. M., Osman, A., Elsayed, M., and Monsour, H.: Genetic improvement of some productive traits in Zaraibi goats, Arab Univ. J. Agric. Sci., 28, 207–216, 2020.
Alawiansyah, A., Kusminanto, R. Y., Widyas, N., Pramono, A., Cahyadi, M., and Saturno: Phenotypic diversity of five goat populations in tropical environment, Eco. Env. Cons., 26, S100–S105, 2020.
Al-Barzinji, Y. M. S. and Hamad, A. O.: Characterization of local goat breeds using RAP-DNA markers, 6th International conference and workshops on basic and applied sciences, https://doi.org/10.1063/1.5004287, 2017.
Alejandre-Ortiz, M. E., Rubio-Tabárez, E., Pérez-Eguía, E., Zaragoza-Martínez, L., and Rodríguez-Galván, G.: Los recursos caprinos de México, in: Biodiversidad Caprina Iberoamericana, edited by: Vargas Bayona, J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition, Bogotá, Colombia, 94–111, 2016.
Ali Rather, M., Hamdani, A., Ayaz, A., Shanaz, S., Mir, S. A., and Nabi, N.: Morphological, phenotypic, performance traits of nondescript goats in Budgam district of Kashmir, Rum. Sci., 9, 137–140, 2020.
Andrade-Montemayor, H. A.: Producción de caprinos en México. VIII Foro Nacional del Caprino, Tierras Caprino, 18, 24–27, 2017.
Arangurén-Méndez, J. A., Román-Bravo, R., Isea, W., Villasmil, Y., and Jordana, J.: Los microsatélites (STR's), marcadores moleculares de ADN por excelencia para programas de conservación: Una revisión, Arch. Lat. Prod. Anim., 13, 30–42, 2005.
Argyriadou, A., Gelasakis, A. I., Banos, G., and Arsenos, G.: Genetic improvement of indigenous Greek sheep and goat breeds, J. Hell. Vet. Med. Soc., 71, 2063–2072, https://doi.org/10.12681/jhvms.22967, 2020.
Ariyaranthe, H. B. P., Ariyaranthe, H. B., and Lokugalappatti, L. G.: Analysis of genetic structure of non-descript local goat populations in Sri Lanka, Proceedings of the Peradeniya University International Research Sessions XX, 4–5 November 2016, Sri Lanka, https://www.researchgate.net/publication/326096026 (last access: 5 August 2020), 2016.
Ariyaranthe, H. B. P., Ariyaranthe, H. B. S., and Lokugalappatti, L. G.: Single nucleotide polymorphism of candidate genes in non-descript local goats of Sri Lanka, Livest. Sci., 196, 49–54, https://doi.org/10.1016/j.livsci.2016.12.012, 2017.
Atoui, A., Carabaño, M. J., and Najari, S.: Evaluation of a local goat population for fertility traits aiming at the improvement of its economic sustainability through genetic selection, Spanish J. Agric. Res., 16, e0404, https://doi.org/10.5424/sjar/2018162-12604, 2018.
Atoui, A., Carabaño, M. J., Abdennebi, M., and Najari S.: Impact of simplified methods of growth recording on genetic parameter estimates of Tunisian local goat population under a low input production system, Ital. J. Anim. Sci., 19, 222–229, https://doi.org/10.1080/1828051X.2020.1720531, 2020.
Bambou, J. C., Vachiery, N., Despois, P., Giraud-Girard, K., Arquet, R., Pinarello, V., Aprelon, R., Barbier, C., Gobardham, J., Mandonnet, N., and Lefrancois, T.: Assessment of genetic variability of resistance to heartwater in Creole goats, Adv. Anim. Biosci., 1, 408–409, https://doi.org/10.1017/S2040470010000361, 2010.
Bambou, J. C., Larcher, T., Ceï, W., Dumoulin, P. J., and Mandonnet, N.: Effect of experimental infection with Haemonchus contortus on parasitological and local cellular responses in resistant and susceptible young Creole goats, BioMed. Res. Int., 902759, https://doi.org/10.1155/2013/902759, 2013.
Bedada, A. E., Gilo, B. N., and Debela, G. T.: Morphometric and physical characterization of Borana Indigenous goats in Southern Oromia, Ethiopia, Univ. J. Agric. Res., 7, 25–31, https://doi.org/10.13189/ujar.2019.070104, 2019.
Bensaid, A., Camus, E., Depre, E., Maillard, J.C., Matheron, G., Pepin, L., and Ruff, G.: Resistance or tolerance of animals to disease and veterinary epidemiology and diagnostic methods, edited by: Uilenberg, G. and Hamers, R., 30–34, Cirad, Maisons-Alfort, France, 1993.
Benyoub, K., Ameur, A. A., and Gaouar, S. B. S.: Phenotypic characterization of local goats populations in western Algerian: Morphometric measurements and milk quality, Gen. Biodiv. J., 2, 73–80, 2018.
Bertaglia, M., Mormont, M., and Trometter, M.: Conserving local goat's breeds and traditional pastoralism in Southern France, Agric. Medit., 135, 77–94, 2005.
Bhuiyan, M. S. A., Haque-Bhuiyan, A. K. F., Lee, J. H., and Lee, S. H.: Community based livestock breeding programs in Bangladesh: Present Status and Challenges, J. Anim. Breed. Genom., 1, 77–84, https://doi.org/10.12972/jabng.20170009, 2017.
Biscarini, F., Nicolazzi, E. L., Stella, A., Boettcher, P. J., and Gandini, G.: Challenges and opportunities in genetic improvement of local livestock breeds, Front. Genet., 6, 1–7, https://doi.org/10.3389/fgene.2015.00033, 2015.
Bishop, S. C. and Morris, C. A.: Genetics of disease resistance in sheep and goats, Small Rum. Res., 70, 48–59, https://doi.org/10.1016/j.smallrumres.2007.01.006, 2007.
Boettcher, P. J., Hoffmann, I., Baumung, R., Drucker, A. G., McManus, C., Berg, P., Stella, A., Nilsen, L. B., Moran, D., Naves, M., and Thompson, M. C.: Genetic resources and genomics for adaptation of livestock to climate change, Front. Genet., 5, 461, https://doi.org/10.3389/fgene.2014.00461, 2014.
Braga-Lôbo, R. N., Facó, O., Lôbo, A. M., and Vasques Villela, L. C.: Brazilian goat breeding programs, Small Ruminant Res., 89, 149–154, https://doi.org/10.1016/j.smallrumres.2009.12.038, 2010.
Buhari, H. U., Saidu, S. N. A., Kudi, C. A., Okocha, E. C., and Katungo, B. Y.: Seroprevalence of Brucella infection in small ruminants from two institutional farms and a slaughter slab in Zaria, Nigeria, Sokoto J. Vet. Sci., 18, 91–99, https://doi.org/10.4314/sokjvs.v18i2.5, 2020.
Ceccobelli, S., Lasagna, E., Demir, E., Roveli, G., Albertini, E., Veronesi, F., Sarti, F. M., and Rosellini, D.: Molecular identification of the “Facciuta Della Valnerina” local goat population reared in the Umbria region, Italy, Animals, 10, 601, https://doi.org/10.3390/ani10040601, 2020.
Chacón, E., Macedo, F., Velázquez, F., Rezende Paiva, S., Pineda, E., and McManus, C.: Morphological measurements and body indices for Cuban Creole goats and their crossbreds, Rev. Bras. Zootecn., 40, 1671–1679, https://doi.org/10.1590/S1516-35982011000800007, 2011.
Chacón-Marcheco, E., La O Arias, M., Fonseca-Fuentes, N., Pérez-Pineda, E., Velázquez-Rodríguez, F. J., Coss-Domínguez, Y., Fonseca-Jiménez, Y., Delgado-Bermejo, J. V., and Martínez-Martínez, A.: Caracterización genética y conservación de la cabra criolla cubana, in: Biodiversidad Caprina Iberoamericana, edited by: Vargas Bayona J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition, Bogotá, Colombia, 112–129, 2016.
Costa, C. A. F., da Silva Vieira, L., Berne, M. E. A., Silva, M. U. D., Guidoni, A. L., and Figueiredo, E. A. P.: Variability of resistance in goats infected with Haemonchus contortus in Brazil, Vet. Parasitol., 88, 153–158, https://doi.org/10.1016/s0304-4017(99)00207-1, 2000.
Dadousis, C., Cecchi, F., Ablondi, M., Fabri, M. C., Stella, A., and Bozzi, R.: Keep Garfagnina alive. An integrated study on patterns of homozygosity, genomic inbreeding, admixture and breed traceability of the Italian Garfagnina goat breed, Plos One, 16, e0232436, https://doi.org/10.1371/journal.pone.0232436, 2021.
Dagong, M. I. A., Bugiwati, S. R. A., Rahim, L., and Purnomo, N.: Genetics polymorphisms of INHA in local goat populations in south-west Sulawesi region of Indonesia, American J. Anim. Vet. Sci., 15, 43–47, https://doi.org/10.3844/ajavsp.2020.43.47, 2020.
de la Chevrotière, C., Moreno, C., Jaquiet, P., and Mandonnet, N.: La sélection génétique pour la maîtrise des strongyloses gastro-intestinales des petits ruminants, INRA Prod. Anim., 24, 221–234, 2011.
Deniskova, T. E., Dotsev, A. V., Selionova, M. I., Reyer, H., Sölkner, J., Fornara, M. S., Aybazov, A. M., Wimmers, K., Brem, G., and Zinovieva N.: SNP-Based genotyping provides insight into the west Asian origin of Russian local goats, Front. Genet., 12, 708740, https://doi.org/10.3389/fgene.2021.708740, 2021.
Deza, C.: Los caprinos criollos como base del mejoramiento genético en ambientes agroecológicamente restrictivos, XIV Mesa Caprina Nacional, Salta, Argentina, 28 pp., 2007.
Di Gerlando, R., Mastrangelo, S., Moscarelli, A., Tolone, M., Sutera, A. M., Portolano, B., and Sardina, M. T.: Genomic structural diversity in local goats: Analysis of copy-number variations, Animals, 10, 1040, https://doi.org/10.3390/ani10061040, 2020.
Dinkisa, G.: Review on control of cowdriosis in ruminants, Int. J. Vet. Sci. Tech., 3, 13–19, 2018.
Djemali, M.: Genetic improvement objectives of sheep and goats in Tunisia: Lessons Learned, in: Analysis and definition of the objectives improvement programmes in sheep and goats. An economic approach to increase their profitability, edited by: Gabiña, D., Options Méditerranéenes: Serie A, Séminaires Mediterranënes, 43, 121–127, 2000.
Dominik, S.: Quantitative trait loci for internal nematode resistance in sheep: A review, GSE, 37, 83–96, https://doi.org/10.1186/1297-9686-37-S1-S83, 2005.
Dorantes-Coronado, E. J., Torres-Hernández, G., Hernández-Mendo, O., and Rojo-Rubio, R.: Zoometric measures and their utilization in prediction of live weight of local goats in southern México, SpringerPlus, 4, 695, https://doi.org/10.1186/s40064-015-1424-6, 2015.
Du, L., Li, J., Ma, N., Ma, Y., Wang, J., Yin, C., and Fu, C.: Animal genetic resources in china: sheep and Goats, Agriculture Press, 229 pp., https://doi.org/10.1017/S2078633612000604, 2011.
Dubeuf, J. P. and Boyazoglu, J.: An international panorama of goat selection and breeds, Livest. Sci., 120, 225–231, https://doi.org/10.1016/j.livsci.2008.07.005, 2009.
El Hag, F. M., Tsubo, M., Rekik, M., Haile, A., Getachew, T., Hilali, M., Khatir, A. A., Babiker, I. E. A., Mussa, A. I., Ahmed, M. K. A., El Hag, M. G., and Zakieldeen, S. A.: Goat breeding objectives in relation to agroecological zonation under dryland farming conditions of north Kordofan, Sudan, World J. Agric. Soil Sci., 5, 1–7, https://doi.org/10.33552/wjass.2020.05.000604, 2020.
El Moutchou, N., González, A. M., Chentouf, M., Lairini, K., and Rodero, E.: Morphological differentiation of Northern Morocco goat, J. Livest. Sci. Tech., 5, 33–41, https://doi.org/10.22103/jlst.2017.1662, 2017.
Escareño-Sánchez, L. M., Wurzinger, M., Pastor-López, F., Salinas-González, H., Solkner J., and Iñiguez, L.: La cabra y los sistemas de producción caprina de los pequeños productores de la Comarca Lagunera en el norte de México, Revista Chapingo Serie Ciencias Forestales y del Ambiente, 17, 235–246, 2011.
FAOSTAT: Producción de caprinos en el mundo, https://www.fao.org/faostat/es/#data/QCL (last access: 5 April 2021), 2019.
Gaddour, A., Najari, S., and Ouni, M.: Response to absorption of the crossbreeding of the local goat with exotic breeds in the oases of southern Tunisia, Afr. J. Agric. Res., 5, 363–371, 2010.
Gandini, G., Turri, F., Rizzi, R., Crotta, M., Minozzi, G., and Pizzi, F.: Economic evaluation of genetic improvement in local breeds: the case of the Verzaschee goat, Ital. J. Anim. Sci., 17, 199–207, https://doi.org/10.1080/1828051X.2017.1279034, 2017.
García-Bonilla, D. V., Vargas-López, S., Bustamante-González, A., Torres-Hernández, G., Calderón-Sánchez, F., and Olvera-Hernández, J. I.: La producción de caprinos para carne en la montaña de Guerrero, México, Agr. Soc. Des., 15, 1–17, 2018.
Ginja, C., Gama, L. T., Martínez, A., Sevane, N., Martin-Burriel, I., Lanari, M. R., Revidatti, M. A., Aranguren-Méndez, J. A., Bedotti, D. O., Ribeiro, M. N., Sponenberg, P., Aguirre, E. L., Alvarez-Franco, L. A., Menezes, M. P. C., Chacón, E., Galarza, A., Gómez-Urviola, N., Martínez-López, O. R., Pimenta-Filho, E. C., da Rocha, L. L., Stemmer, A., Landi, V., and Delgado-Bermejo, J. V.: Genetic diversity and patterns of population structure in Creole goats from the Americas, Anim. Genet., 48, 315–329, https://doi.org/10.1111/age.12529, 2017.
Giovannini, N.: Evaluación y selección de reproductores para la mejora genética, INTA, Bariloche, Argentina, 13 pp., https://inta.gob.ar/sites/default/files/script-tmp-inta-evaluacion_y_seleccion_de_reproductores_para_mej.pdf (last access: 13 June 2020), 2011.
Gomes de Lima, L., De Souza, N. O. B., Rios, R. R., de Melo, B. A., dos Santos, L. T. A., Silva, K. M., Murphy, T. W., and Fraga, A. B.: Advances in molecular genetic techniques applied to selection for litter size in goats (Capra hircus): a review, J. Appl. Anim. Res., 48, 38–44, https://doi.org/10.1080/09712119.2020.1717497, 2020.
Gómez-Carpio, M. M., Toalombo-Vargas, P. A., Avilés-Esquivel, D. F., Mendoza, B., Pesántez, M., Vargas, J. C., and Aguirre, L.: Recursos genéticos caprinos locales en el Ecuador, in: Biodiversidad Caprina Iberoamericana, edited by: Vargas Bayona J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition, Bogotá, Colombia, 149–160, 2016.
Gómez-Urviola, N. C., Gómez-Urviola, J. W., Celi-Mariátegui, I. D. R., Milán-Sendra, M. J., and Jordana-Vidal, J.: La cabra criolla peruana, situación actual y perspectivas conservacionistas, Biodiversidad Caprina iberoamericana, edited by: Vargas Bayona J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition, Bogotá, Colombia, 163–168, 2016.
Gunia, M., Mandonnet, N., Arquet, R., de la Chevrotiere, C., Navès, M., Mahieu, M., and Alexandre G.: Production systems of Creole goat and their implications for a breeding programme, Animal, 4, 2099–2105, https://doi.org/10.1017/S1751731110001412, 2010.
Hagan, J. K., Apori, S. O., Bosompem, M., Ankobea, G., and Mawuli, A.: Mophological characteristics of Indigenous goats in the coastal savanna and forest eco-zones of Ghana, J. Anim. Sci. Adv., 2, 813–821, 2012.
Haile, A., Getachew, T., Mirkena, T., Duguma, G., Gizaw, S., Wurzinger, M., Soelkner, J., Mwai, O., Deesie, T., Abebe, A., Abate, Z., Jembere, T., Rekik, M., Lobo, R. N. B., Mwacharo, J. M., Terfa, Z. G., Kassie, G. T., Mueller, J. P., and Richkowsky, B.: Community-based sheep breeding programs generated substantial genetic gains and socioeconomic benefits, Animal, 14, 1362–1370, https://doi.org/10.1017/S1751731120000269, 2020.
Hankamo, A., Woldeyohannes, T., and Banerjee, S.: Morphometrical characterization and structural indices of Indigenous goats reared in two production systems in Sidama Zone, Southern Ethiopia, Int. J. Anim. Sci. Tech., 4, 6–16, https://doi.org/10.11648/j.ijast.20200401.12, 2020.
Hassen, H., Rishkowsky, B., Termanini, A., Jessry, G., Haile, A., Baum, M., and Lababidi, S.: Mophological and molecular genetic diversity of Syrian indigenous goat populations, Afr. J. Biotechnol., 15, 745–758, https://doi.org/10.5897/AJB2015.15062, 2016.
Hilal, B., El Otmani, S., Chentouf, M., and Boujenan, I.: Multivariate analysis for morphological traits of the Hamra goat population in two regions of Morocco, Anim. Genet. Resour., 59, 55–62, https://doi.org/10.1017/S2078633616000114, 2016.
Iñiguez-Rojas, L., Mueller, J. P., Facó, O., Wurzinger, M., Sölkner, J., Rodríguez, T., and Salinas-González, H.: Limitaciones y sostenibilidad del mejoramiento genético comunitario para pequeños productores en las zonas áridas de Latinoamérica, in: La Producción de Rumiantes Menores en las Zonas Áridas de Latinoamérica, edited by: Iñiguez-Rojas, L., 515–538, 2013.
Jayashree, R., Jayashankar, M. R., Nagaraja, C. S., Shikrishna, I., and Satyanarayana, K.: Genetic characterization of local goats of Karnakata by microsatellite marker analysis, Ind. J. Anim. Res., 53, 19–23, https://doi.org/10.18805/ijar.B-3458, 2019.
Jemmali, B., Kamoun, M., Haddar, M., Ben Gara, A., Selmi, H., Hammi, M., Amraoui, M., Rouiddi, H., and Boulbaba, R.: Genetic polymorphism of casein alpha-S1 gene in Tunisian local goat, Biomirror, 3, 1–4, 2012.
Karnuah, A. B., Dunga, G., and Rewe, T.: Community based breeding program for improve goat production in Liberia, MOJ Curr. Res. Rev., 1, 216–221, 2018a.
Karnuah, A. B., Osei-Amponsah, R., Dunga, G., Wennah, A., Wiles, W. T., and Boettcher, P.: Phenotypic characterization of the West Africa dwarf goats and the production system in Liberia, Int. J. Livest. Prod., 9, 221–231, https://doi.org/10.5897/IJLP2018.0496, 2018b.
Karsli, T., Demir, E., Fidan, H. G., Aslan, M., Karsli, B. A., Arik, I. Z., Semerci, E. S., Karabag, K., and Balcioglu, M. S.: Determination of genetic variability, population structure and genetic differentiation of indigenous Turkish goat breeds based on SSR loci, Small Ruminant. Res., 190, 106147, https://doi.org/10.1016/j.smallrumres.2020.106147, 2020.
Kaumbata, W., Nakimbugwe, H., Nandolo, W., Banda, L. J., Mészáros, G., Gondwe, T., Woodward-Greene, M. J., Rosen, B., Van-Tassel, C. P., Sölkner, J., and Wurzinger, M.: Experiences from the implementation of Community-Based goat breeding programs in Malawi and Uganda: A potential approach for conservation and improvement of Indigenous small ruminants in smallholder farms, Sustainability, 13, 1494, https://doi.org/10.3390/su13031494, 2021.
Kim, J.-Y., Jeong, S., Kim, K. H., Lim, W.-J., Lee, H.-Y., and Kim, N.: Discovery of Genomic Characteristics and Selection Signatures in Korean Indigenous Goats Through Comparison of 10 Goat Breeds, Front. Genet., 10, 699, https://doi.org/10.3389/fgene.2019.00699, 2019.
Kosgey, I. S., Baker, R. L., Udo, H. M. J., and Van Aredonk, J. A. M.: Successes and failures of small ruminant breeding programmes in the tropics: a review, Small Ruminant Res., 61, 13–28, https://doi.org/10.1016/j.smallrumres.2005.01.003, 2006.
Labatut, J., Girard, N., Astruc, J. M., and Bibe, B.: Dissemination of genetic progress: A key aspect of genetic improvement of local breeds, Anim. Genet. Resour., 53, 117–127, https://doi.org/10.1017/S2078633612000367, 2013.
Lanari, M. R., Domingo, E., Pérez-Centeno, M. J., and Gallo, L.: Pastoral community selection and the genetic structure of local goat breed in Patagonia, Anim. Genet. Resour., 37, 31–42, https://doi.org/10.1017/S1014233900001942, 2005.
Liu, G., Zhao, Q., Lu, J., Sun, F., Han, X., Zhao, J., Feng, H., Wang, K., and Liu, C.: Insights into the genetic diversity of indigenous goats and their conservation priorities, Asian-Aust. J. Anim. Sci., 32, 1501–1510, https://doi.org/10.5713/ajas.18.0737, 2019.
Lourenco, D., Legarra, A., Tsuruta, S., Masuda, Y., Aguilar, C. and Misztal, I.: Single-Step genomic evaluations from theory to practice: Using SNP chips and sequence data in BLUPF90, Genes, 11, 790, https://doi.org/10.3390/genes11070790, 2020.
Lozano-Jaramillo, M., Alemu, S. W., Dessie, T., Komen, H., and Bastiaansen, J. W. M.: Using phenotypic distribution models to predict livestock performance, Sci. Rep.-UK, 9, 15371, https://doi.org/10.1038/s41598-019-51910-6, 2019.
Maldonado-Jáquez, J. A., Salinas-González, H., Torres-Hernández, G., Becerril-Pérez, C. M., and Díaz-Rivera, P.: Factors influencing milk production in local goats in the Comarca Lagunera, México, LRRD, 30, 132, http://www.lrrd.org/lrrd30/7/glat30132.html (last access:last access: 12 October, 2020), 2018.
Mandal, A., Karunakaran, M., Rout, P. K., and Roy, R.: Conservation of threatened goat breeds in India, Anim. Genet. Res., 55, 47–55, https://doi.org/10.1017/S2078633614000307, 2014.
Mandonnet, N., Aumont, G., Fleury, J., Arquet, R., Varo, H., Gruner, L., Bouix, J., and Vu Tien Khang, J.: Assessment of genetic variability of resistance to gastrointestinal nematode parasites in Creole goats in the humid tropics, J. Anim. Sci., 79, 1706–1712, https://doi.org/10.2527/2001.7971706x, 2001.
Mandonnet, N., Mahieu, M., Alexandre, G., Gunia, M., and Bambou, J. C.: Genetic resistance to parasites in small ruminants: from knowledge to implementation in the tropics, in: Proceedings 10th World Congress on Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August, 2014, 1–6, 2014.
Mariante, A. S., Albuquerque, M. S., Egito, A. A., and McManus, C.: Advances in the Brazilian animal genetic resources conservation programme, Anim. Genet. Resour., 25, 107–121, https://doi.org/10.1017/S1014233900003497, 1999.
Matheron, G., Barré, N., Camus, E., and Gogue, J.: Genetic resistance of Guadeloupe Native goats to heartwater, Onderstepoort J. Vet. Res., 54, 337–340, 1987.
Menezes, M. P., Martinez, A. M., Pimenta, E. C., Vega-Pla, J. L., Delgado, J. V., Arandas, J. K., da Rocha, L. L., and Ribeiro, M. N.: Diversity and genetic relationship among local Brazilian goat breeds using SSR markers, Animals, 10, 1842, https://doi.org/10.3390/ani10101842, 2020.
Meydan, H., Pehlivan, E., Özkan, M. M., Yildiz, M. A., and Goldman, W.: Prion protein gene polymorphisms in Turkish native goat breeds, J. Genet., 96, 299–305, https://doi.org/10.1007/s12041-017-0763-1, 2017.
Michailidou, S., Tsangaris, G. T., Tzora, A., Skoufos, I., Banos, G., Argiriou, A., and Arsenos, G.: Analysis of genome-wide DNA arrays reveals the genomic population structure and diversity in autochthonous Greek goat breeds, PLoS ONE, 14, e0226179, https://doi.org/10.1371/journal.pone.0226179, 2019.
Migliore, S., Agnello, S., Chiappini, B., Vaccari, G., Mignacca, S. A., Presti, V. D. M. L., Di Domenico, F., and Vitale, M.: Biodiversity and selection for scrapie resistance in goats: genetic polymorphism in “Girgentana” breed in Sicily, Italy, Small Ruminant Res., 125, 137–141, https://doi.org/10.1016/j.smallrumres.2015.01.029, 2015.
Miller, J. E. and Horohov, D. W.: Immunological aspects of nematode parasite control in sheep, J. Anim. Sci., 84, E124–E132, https://doi.org/10.2527/2006.8413_supple124x, 2006.
Monau, P., Raphaka, K., Zvinorova-Chimboza, P., and Gondwe, T.: Sustainable utilization of Indigenous goats in Southern Africa, Diversity, 12, 20, https://doi.org/10.3390/d12010020, 2020.
Montaldo, H. H., Torres-Hernández, G., and Valencia-Posadas, M.: Goat breeding research in México, Small Ruminant Res., 89, 155–163, https://doi.org/10.1016/j.smallrumres.2009.12.039, 2010.
Moyao-Ariza, F., Maldonado-Jáquez, J. A., Granados-Rivera, L. D., Martínez-Rojero, R. D., Torres-Hernández, G., Domínguez-Martínez, P. A., Bautista-Martínez, Y., and Sánchez-Gutiérrez, R. A.: Variabilidad morfoestructural, zoométrica y faneróptica de machos cabríos locales del norte de México, ITEA, https://doi.org/10.12706/itea.2021.030, online first, 2022.
Mrode, R., Tarekegn, G. M., Mwacharo, J. M., and Djikeng, A.: Invited review: Genomic selection for small ruminants in developed countries: how applicable for the rest of the world?, Animal, 12, 1333–1340, https://doi.org/10.1017/S1751731117003688, 2018.
Mrode, R., Dzivenu, C. E., Marshall, K., Gibson Chagunda, M. G., Muasa, B. S., Ojango, J., and Okeyo, A. M.: Phenomics and its potential impact on livestock development in low-income countries: innovative applications of emerging related digital technology, Anim. Front., 10, 1–6, https://doi.org/10.1093/af/vfaa002, 2020.
Mueller, J. P.: Programas de mejora genética de rumiantes menores basados en comunidades, Arch. Lat. Prod. Anim., 25, 61–75, 2017.
Naves, M., Alexandre, G., Mahieu, M., Bambou, J.C., Gunia, M., de la Chevrotiere, C., Limea, L., Nizar, S., Menendez-Buxadera, A., Jannini, D., and Mandonnet, N.: La cabra creole de las Antillas Francesas y Haití: un recurso genético original y productivo para el desarrollo de sistemas de producción diversificados, in: Biodiversidad Caprina Iberoamericana, edited by: Vargas Bayona, J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition. Bogotá, Colombia, 112–129, 2016.
Nwogwugwu, C., Lee, S. H., Freedom, E. C., Manjula, P., and Lee, J. H.: Review on challenges, opportunities and genetic improvement of sheep and goat productivity in Ethiopia, J. Anim. Breed. Genom., 2, 001–008, https://doi.org/10.12972/jabng.20180015, 2018.
Ojo, O. A., Akpa, G. N., Orunmuyi, M., and Adeyinka, I. A.: Genetic differentiation among Nigerian Indigenous goat populations, J. Agric. Sci., 7, 39–47, https://doi.org/10.5539/jas.v7n11p39, 2015.
Onzima, R. B., Upadhyay, M. R., Doekes, H. P., Brito, L. F., Bosse, M., Kanis, E., Groenen, M. A. M., and Crooijmans, R. P. M. A.: Genome-wide characterization of selection signatures and runs of homozygosity in Ugandan goat breeds, Front. Genet., 9, 318, https://doi.org/10.3389/fgene.2018.00318, 2018.
Ortiz-Morales, O., Arias-Margarito, L., López-Ojeda, J.C., Soriano-Robles, R., Almaraz-Buendia, I., Ramírez-Bibriesca E. Estudio descriptivo de la producción caprina tradicional en las regiones mixteca y valles centrales de Oaxaca, México, Ecosist. Rec. Agrop., 8, e2840, https://doi.org/10.19136/era.a8n2.2840, 2021.
Paim, T. P., Faria, D. A., Hay, E. H., Lanari, R., Chaverri-Esquivel, L., Cascante, M. I., Jimenez-Alfaro, E., Mendez, A., Faco, O., de Moraes Silva, K., Mezzadra, C. A., Mariante, A., Rezende-Paiva, S., and Blackburn, H. D.: New world populations are a genetically diverse reservoir for future use, Sci. Rep.-UK, 9, 1476, https://doi.org/10.1038/s41598-019-38812-3, 2019.
Palomares-Reséndiz, G., Aguilar-Romero, F., Flores-Pérez, C., Gómez-Núñez, L., Gutierrez-Hernández, J., Herrera-López, E., Limón-González, Morales-Álvarez, F., Pastor-López, F., and Díaz-Aparicio, E.: Enfermedades infecciosas de relevancia en la producción caprina, histora, retos y perspectivas, Rev. Mex. Cienc. Pecu., 12, 205–223, https://doi.org/10.22319/rmcp.v12s3.5801, 2021.
Patbandha, T. K., Pata, B. A., Trivedi, S. P., Gohil, P. C., Boradiya, P. C., Sharma, A., and Savalia, K. B.: Evaluating phenotypic correlation between body weight and biometric traits of migratory goats, J. Entom. Zool. Stud., 6, 560–564, 2018.
Pogorevc, N., Simčič, M., Khayatzadeh, N., Sölkner, J., Berger, B., Bojkovski, D., Zorc, M., Dovc, P., Medugorac, I., and Horvat, S.: Post-genotyping optimization of dataset formation could affect genetic diversity parameters: an example of analyses with alpine goat breeds, BMC Genomics, 22, 546, https://doi.org/10.1186/s12864-021-07802-z, 2021.
Preston, S. J. M., Sandeman, M., González, J., and Piedrafita, D.: Current Status for gastrointestinal nematode diagnosis in small ruminants: Where are and where are we going?, J. Immun. Res., 2014, 210350, https://doi.org/10.1155/2014/210350, 2014.
Prieto, P. N., Revidatti, M. A., Capellari, A., and Ribeiro, M. N.: Estudio de recursos genéticos: identificación de variables morfoestructurales en la caracterización de los caprinos nativos de Formosa, Comunicaciones Científicas y Tecnológica, Argentina, 2006.
Reveles-Torres, L. R., Echavarría, C. H. F., Bañuelos, V. R., Salinas-González, H., and Cabral, A. F. J.: Empleo de marcadores moleculares en la diferenciación de razas caprinas del Estado de Zacatecas, México, Trop. Subtrop. Agroec., 9, 15–27, 2008.
Rewe, T. O., Ogore, P. B., and Kahi, A. K.: Integrated goat projects In Kenya: Impact on genetic improvement, 7th World Congress on Genetics Applied to Livestock Production, 19–23 August 2002, Montpellier, France, 19–23 August 2002, 2002.
Rotimi, E. A., Momoh, O. M., and Egahi, J. O.: Relationship between bodyweight and morphological traits in Sahelian goats of Nigeria using path analysis, Mustafa Kemal University J. Agric. Sci., 25, 455–460, https://doi.org/10.11648/j.ijast.20200401.12, 2020.
Saatci, M., Elmaz, Ö., Akbas, A. A., Agaoglu, Ö, K., Sari, M., and Metin, M. Ö.: Some effects of nationwide small ruminant breeding project under the breeder conditions on goat flocks and their owner, Rev. Agric. Rural Develop., 6, 5–9, 2017.
Sahraoui, H., Madani, T., Fantazi, K., Khouane, A. C., Ameur, A. A., Paschinno, P., Vacca, G. M., Gaouar, S. B., and Detorri, M. L.: Genetic variability in the A microsatellite al SLC11A1 gene and possible implications with innate resistance against brucelosis in Algerian native goats, Biodiversitas, 21, 5630–5636, https://doi.org/10.13057/biodiv/d211219, 2020.
Salinas-González, H., Echavarría, F. G., Flores-Nájera, M. J., Flores-Ortiz, M. A., Gutiérrez, R., Rumayor, A., Meza-Herrera, C. A., and Pastor, F.: Evaluación participativa de tecnologías en caprinos en el semiárido del Norte Centro de México, Revista Chapingo-Serie Ciencias Forestales y del Ambiente, 17, 225–234, https://doi.org/10.5154/r.rchscfa.2010.11.106, 2011.
Salinas-González, H., Flores-Nájera, M. J., Echavarría-Chairez, F., and Meza-Herrera, C. A.: Investigación participativa y su rol en el desarrollo y la investigación de rumiantes menores en zonas áridas de México, in: La Producción de Rumiantes Menores en las Zonas Áridas de Latinoamérica, ISBN 978-85-7035-229-3, edited by: Iñiguez-Rojas, L., 11, 249–277, 2013.
Sánchez-Gutiérrez, R. A., Gutiérrez-Luna, R., and Flores-Nájera, M. J.: Caracterización morfológica de un rebaño de conservación de cabras criollas en Zacatecas, México, Arch. Zoot., 67, 73–79, 2018.
Sánchez-Gutiérrez, R. A., Granados-Rivera, L. D., Salinas-González, H., Maldonado-Jáquez, J. A., Hernández-Leal, E., and Cigarroa-Vázquez, F. A.: Selección preliminar de cabras Blanca Celtibérica mediante una técnica multivariada, Zootecnia Tropical, 39, e4484416, https://doi.org/10.5281/zenodo.4484415, 2021.
Schultz, E. B., Silva, F. F., Garcia, A. O., Oliveira, H. R., Rodrigues, M. T., and Brito, L. F.: Genetic parameter estimates for caprine arthritis encephalitis in dairy goats, J. Dairy Sci., 103, 6407–6411, https://doi.org/10.3168/jds.2019-17740, 2020.
Sevane, N., Cortés, O., Gama, L. T., Martínez, A., Zaragoza, P., Amills, M., Bedotti, D. O., Bruno de Sousa, C., Cañon, J., Dunner, S., Ginja, C., Lanari, M. R., Landi, V., Sponenberg, P., Delgado, J. V., and BioGoat Consortium: Dissection of ancestral contributions to creole goat populations, Animal, 12, 2017–2026, https://doi.org/10.1017/S1751731117003627, 2018.
Silva, F. F., Bambou, J. C., Oliveira, J. A., Barbier, C., Fleury, J., Machado, T., and Mandonnet, N.: Genome wide association study reveals new candidate genes for resistance to nematodes in Creole goat, Small Ruminant. Res., 166, 109–114, https://doi.org/10.1016/j.smallrumres.2018.06.004, 2018.
Stemmer, A. and Valle-Zárate, A.: La crianza de caprinos en Bolivia y la función primordial de la cabra criolla, in: Biodiversidad Caprina Iberoamericana, edited by: Vargas Bayona, J. E., Zaragoza Martínez, L., Delgado Bermejo, J. V., Rodríguez Galván, G., 1st. Edition. Bogotá, Colombia., 167–186, 2016.
Tantia, M. S., Vij, P. K., Yathish, H. M., Kulkarni, V. S., Shettar, V. B., Gopala, G. T., Sharma, H., and Sharma R.: Characterization of Nandidurga and Bidri goat populations of Karnataka, Indian J. Anim. Sci., 88, 1050–1063, 2018.
Teklu, G., Aman, E., Enbiyale, G., Masrie, O., Neges, T., Yirga, A., Debalke, D., and Mitku, F.: Review on cowdriosis (heartwater), J. Am. Sci., 3, 99–106, https://doi.org/10.7537/marsjas130817.12, 2017.
Torres-Acosta, J. F. J., Jacobs, D. E., Aguilar-Caballero, A. J., Sandoval-Castro, C., Cob-Galera, L., and May-Martínez, M.: Improving resilience against natural gastrointestinal nematode infections in browsing kids during the dry season in tropical México, Vet. Parasitol., 135, 163–173, https://doi.org/10.1016/j.vetpar.2005.08.009, 2006.
Torres-Hernández, G., Maldonado-Jáquez, J. A., Salinas-González, H., Granados-Rivera, L. D., Pastor-López, F. J., Vaquera-Huerta, H., and Bautista-Martínez, Y.: Influence of milk production records on the estimation of typical and extended curve lactation parameters in local goats under grazing management, Indian J. Anim. Sci., 90, 164–167, 2020.
Tyasi, T. L., Mathapo, M. C., Mokoena, K., Maluleke, D., Rashijane, L. T., Makgowo, K. M., Danguru, L. W., Molabe, K. M., Bopape, P. M., and Mathye, N. D.: Assesment of relationship between body weight and morphological traits of South African Non-descript Indigenous goats, J. Anim. Health Prod. 8, 32–39, 2020.
Valencia-Posadas, M., Barboza-Corona, J. E., Ángel-Sahagun, C. A., Gutiérrez-Chávez, A. J., Martínez-Jaime, O. A., and Montaldo, H. H.: Phenotypic correlations between milk production and conformation in goats, Acta Universitaria, 28, 3–8, https://doi.org/10.15174/au.2017.1093, 2017.
Villarreal-Arellano, H. R., Fuentes-Mascorro, G., Ramírez-Bibriesca, E., Torres-Hernández, G., Ricardi de la Cruz, C., and Vargas-López, S.: Morphostructural variability in the Pastoreña goat in different regions of the Mixteca of Mexico: A phenotypic study to establish the racial profile, Rev. Fac. Cienc. Agrar.-UNCuyo, 52, 360–375, 2020.
Visser, C. and van Marle-Köster, E.: The development and genetic improvement of South African goats, Goat Science, Chapter 2, edited by: Sándor Kukovics, IntechOpen, 19–36, https://doi.org/10.5772/intechopen.70065, 2017.
Wattiaux, M. A.: Desafíos de la agricultura ganadera frente al cambio climático, in: Experiencias Ganaderas, Agrícolas y Forestales en la Conservación de los Recursos Naturales, edited by: Herrera-Tapia, F., Arteaga-Reyes, T., Estrada-Flores, J., Escobedo, J. C., and Reyes, J. A., Instituto de Ciencias Agropecuarias y Rurales. Universidad Autonóma del Estado de México y Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. México, 33–47, 2019.
Weldemariam, B. and Mezgebe, G.: Community based small ruminant breeding programs in Ethiopia: progress and Challenges, Small Ruminant. Res., 196, 196264, https://doi.org/10.1016/j.smallrumres.2020.106264, 2021.
Wittenburg, D., Bonk, S., Doschoris, M., and Reyer, H.: Design of experiments for fine-mapping quantitative trait in livestock populations, BMC Genetics, 21, 66, https://doi.org/10.1186/s12863-020-00871-1, 2020.
Wurzinger, M., Gutiérrez, G. A., Sölkner, J., and Probst, L.: Comunnity-Based livestock breeding: Coordinated action or relational process?, Front. Vet. Sci., 8, 613505, https://doi.org/10.3389/fvets.2021.613505, 2021.
Zaman, G., Nahardeka, N., Laskar, S., Das, B., Ferdoci, A. M., Aziz, A., Das, G. C., and Shekar, M. C.: Molecular characterization of Assam local goats using microsatellite markers, Indian Vet. J., 91, 49–52, 2014.
Zein, M. S., Sulandari, S., Muladno, S., and Riwantoro, D.: Genetic diversity and phylogenetic relationship of Indonesian local goats using microsatellite DNA markers, Indonesian J. Anim. Vet. Sci., 17, 25–35, 2012.
- Abstract
- Introduction
- Resistance to parasites and diseases
- Phenotype of local goats as selection criteria in genetic improvement programs
- Use of genomic and molecular tools in local goat breeding
- Challenges, limitations, and opportunities of genetic improvement in local goats
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References
- Abstract
- Introduction
- Resistance to parasites and diseases
- Phenotype of local goats as selection criteria in genetic improvement programs
- Use of genomic and molecular tools in local goat breeding
- Challenges, limitations, and opportunities of genetic improvement in local goats
- Conclusions
- Data availability
- Author contributions
- Competing interests
- Disclaimer
- Acknowledgements
- Review statement
- References





