the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
The sex ratio in farmed American mink (Neovison vison)
Lidia Felska-Błaszczyk
Natalia Ławrów
Bogdan Lasota
Beata Seremak
Katarzyna Pęzińska-Kijak
Krzysztof Żuk
Piotr Nowak
The aim of the study was to analyse the sex ratio of American mink litters in relation to dam's age, gestation length, and time interval between the first and second mating. The observations were carried out on a mink farm located in northern Poland. The analysis involved litters of 207 females, aged 1 (n=107) and 2 years (n=100), which successfully raised all the born kits. The sex of the offspring was identified on weaning. The kits were assigned to groups according to their dam's gestation length, mating date, and first-to-second mating interval. It was found that female kits quantitatively predominated over male offspring. Longer pregnancies, delayed mating time, and greater interval between the first and second mating was accompanied by a higher number of female births in relation to male births.
- Article
(176 KB) - Full-text XML
- BibTeX
- EndNote
Since the mink is a monoestrous species with a pregnancy characterized by embryonic diapause, breeding season is the most demanding time of the year on a production farm both in terms of animal nutrition and human labour. Both planning and implementation of mating design schemes aim to achieve litters as large and strong as possible. The production of the farm is usually supposed to develop and the breeding stock needs to grow. Therefore it is important for the farmer to be aware of sex distribution of the litters of newborn animals. The literature basically lacks reports on sex ratios in herds of farmed American mink, although such information is important for planning prospective cage allocation after weaning. More than 60 years ago, Venge (1953) reported that the sex ratio of litters depended on a number of factors, such as nutrition, heat stage of the mated female, and season. In terms of the productivity of the farm, it is desirable to have the highest possible proportion of females in the offspring. Few literature sources deal with the sex ratio in the American mink, usually referring to natural populations. For example, Sidorovich (1993) observed that relatively more females are born in the Belarusian wild mink populations. Brzeziński et al. (2010) and Craik (2008), on the other hand, observed that more males were caught in cage traps, even twice as many as females, especially in the spring. In autumn, however, the number of caught females was similar to males. Also, Boneset et al. (2006), who observed mink in the natural habitats of the West Estonian archipelago, as well as on the British Isles, found that more males were present than females. The authors concluded that more male mink may be born in the wild, which is also suggested by Zwiernik et al. (2008) and Schüttler et al. (2010). The wild population of the European polecat has also been reported to consist of more males than females (Brzesiński et al., 1992; Barrientos, 2015).
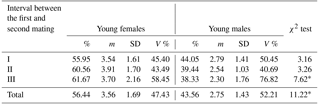
Table 1Sex distribution in newborn mink litters in relation to dams' age and gestation length.
* Differences between the number of females and males per litter significant at the level of P≤0.01. Abbreviations: m – medium, SD – standard deviation, V % – coefficient of variability.
The data collected in the natural habitat do not necessarily reflect the actual sex ratio, as natural nests can be of limited availability or even completely unavailable to researchers, as well as due to differences in male and female behavioural patterns (Székely et al., 2014). Hence the information on this parameter in natural mink populations may be biased. The controlled farm environment, on the other hand, enables adequate and precise determination on the sex ratio of the offspring. The literature, however, lacks reports on this aspect of mink life history, with virtually the only article from more than 60 years ago by Venge (1953), who claimed that the sex ratio in American mink under farming conditions depends on such factors as nutrition, mating term within the heat, or climatic conditions.
Renaville et al. (2001) and Bachtrog et al. (2014) present two general mechanisms of sex determination. An environmental factor acts at the embryonic stage of individual development (some fishes and reptiles). The molecular genetic mechanism, on the other hand, present in birds and mammals, determines the sex on the moment of conception. It is not always easy to designate the physiology processes behind the male-to-female ratio of animal litters (Krackow, 1995a, b). Krackow and Burgoyne (1998) claim that changing the time of mating within the heat in mice will alter the sex ratio in the resulting litters. The authors found out that a female embryo takes more time to develop until blastocyst than a male embryo, and this has a big impact on the time of embryonic implantation in the uterine wall. Monclús et al. (2014) observed an interesting phenomenon; siblings of the opposite sexes may affect one another in their fetal life. The authors found distinct signs of masculinisation of female rabbits and yellow-backed marmots in the litters containing more males than females, which had been caused by excess testosterone. When adult, such females began breeding later compared to those born in more “female” litters. According to Trut (1996) and Price (2002), the population sex ratio is associated with the degree of domestication. For example, silver foxes selected for docility produce more male offspring as compared with a fox population which had not been selected according to disposition. The aim of the study was to analyse sex distribution in mink in relation to the dam's age and the length of the pregnancy from which the litter originates, and in relation to the date of mating and the interval between the first and the second mating.
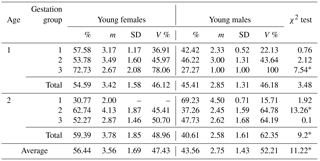
Table 2Sex distribution in newborn mink litters in relation to dams' age and mating date.
* Differences between the number of females and males per litter significant at the level of P≤0.01. Abbreviations: m – medium, SD – standard deviation, V % – coefficient of variability.
The research was carried out on a production farm of American mink located in northern Poland, in the breeding season 2017. Litters from a total of 207 females at the age of 1 (n=107) and 2 years (n=100) were analysed. The sex of the animals was identified on weaning and only 0-mortality litters were included in the study (i.e. all born kits were finally weaned). The data on mating and whelping dates were taken from the farm on-cage records. The same data were used to calculate gestation length for each of the studied litters. The length of gestation ranged from 40 to 69 days, and the offspring were assigned to groups according to gestation length they had been born from:
-
40 to 49 days – group 1,
-
50 to 59 days – group 2,
-
60 to 69 days – group 3.
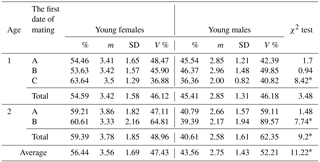
According to the date of the first mating, the offspring were assigned to the following groups:
-
mating between 1 and 5 March – group A,
-
mating between 6 and 10 March – group B,
-
mating between 11 and 15 March – group C.
The following groups were formed according to the first-to-second mating interval:
-
1 day interval – group I,
-
2 days interval – group II,
-
7 days interval – group III.
Statistical analysis included the mean (m), standard deviation (SD) and coefficient of variability (V %). The resulting data were analysed using the STATISTICA 10.0 PL package. The non-parametric chi-squared test was used to evaluate the differences between the number of born males and females.
Table 1 presents the results of the statistical data analysis of females and males born in a litter in relation to dam's age and gestation length. The analysis revealed that generally more females were born than males. On average, older, 2-year-old mink gave birth to more female offspring compared to year-old dams, which is confirmed as significant by the chi-squared test (P≤0.01); nearly 60 % of the offspring were female kits. However, those of year-old dams which went through longest pregnancies produced even more females in a litter, 72 %, 73 % on average, which was significantly the highest percentage (P≤0.01).
Mating date was also significant in relation to sex distribution of the offspring (Table 2), as both 1- and 2-year-old dams mated in the later term (B and C) gave birth to more females, with differences significant at P≤0.01.
Apart from gestation length and mating date, also the first-to-second mating interval seems to have an effect on young mink sex distribution (Table 3); the longer the interval, the higher the proportion of female offspring in the litter, with differences significant at P≤0.01.
Our usual observation on numerous mink farms is that more females are born than males. This has been statistically supported with the analysis presented in this paper. More than 60 years ago, however, Venge (1953), who studied sex ratio in farmed mink populations, reported a slight quantitative domination of males over females. Both Venge (1953) and Sundell (1962) claimed that the mortality of male embryos is higher than that of female embryos. Also Milki et al. (2003) and Orzack et al. (2015) report that in early pregnancy in humans the mortality of male embryos is higher than that of female embryos, despite the fact that more male embryos are conceived, since spermatozoa bearing the Y chromosome are lighter than those with the X, and thus move faster and have a greater chance of reaching the ovum early enough. This may underlie such ample differences in the sex ratios observed in our study. With a longer gestation and – consequently – a longer diapause, male embryo mortality was higher and the litter contained more female offspring, as compared to shorter pregnancies. This has already been observed by Venge (1953), who noticed that more males were born from shorter pregnancies and vice versa. It is difficult, however, to find an answer to the question about the differences between our observations and the data from 60 years ago. Venge (1953) indeed found more males than females in litters. However the differences were not great. Our analysis, on the other hand, revealed that more females are being born at present, and the difference is both higher and statistically significant. Bacon and McClintock (1994) report that the litter sex ratio at birth may be a consequence of several interacting factors. The Trivers–Willard hypothesis (TWH) says that the condition of the parents will bias the sex ratio of the offspring – toward sons when under good conditions and toward daughters when under poor conditions (Keller et al., 2001; Meagher et al., 2012).
Guillon (2016) reached a similar conclusion that the sex ratio is under an influence of the habitat, which directly affects the condition of the animals. Ross (2016) reports that most of sexually reproducing organisms tend to exhibit equilibrium between either sex. According to Bakken (1995, 1998), both litter size and sex ratio in farmed silver foxes are an outcome of the relationships among the animals as well as between the animals and humans, which the author refers to as “social stress”. The author claims that vixens exposed to social stress gave birth to more males. This has been supported by Cameron (2004), who observed that glucose levels increase in socially stressed animals. Krackow and Burgoyne (1998), on the other hand, found that sex ratio in murine litters depends on the moment of mating within the heat cycle; females mated earlier gave birth to more males, whereas those mated later – more females. These results may confirm our data, since 2-year-old female mink, which are mated later in the heat than year-old ones, indeed produced more females in the litter. Different data were presented by Vega et al. (2008), who observed in their studies on rabbits that the time of insemination did not influence the sex ratio of the offspring, although the authors stipulated that the population they experimented upon was not large enough to infer.
Sidorovich (1993), who studied mink in their natural habitat, found that the species is very flexible when it comes to reproduction. Not only are they able to produce large litters, but also more females appear in a litter if the local mink population has for some reason declined. This effect was noticed in Belarus, where excessive hunting pressure led to a significant reduction in the mink population, which in consequence caused that females gave birth to larger litters containing a higher proportion of female offspring. With a strong population, the male-to-female sex ratio was 1:1.1, and the parameter reached 1:1.8 when the population dropped considerably. An analysis of sex population distributions in various animals reported by various authors allows concluding that the ratio is usually 1:1, as confirmed by Ross (2016). It seems the ratio may be altered under farm conditions.
The analysis revealed that more females than males are born in the reproduction of farm mink. Along with a longer pregnancy, a later date of mating, and a larger interval between the first and second mating, the number of female offspring increases in relation to male offspring.
The original data collected are available upon request from the corresponding author.
LFB contributed the general concept, designed the study and supervised each of its stages; LFB and NŁ prepared data sets and performed data analysis; LFB and BL wrote the text of the manuscript; KPK, KZ, and PN collected the data on the farm and provided the study with technical support; NŁ and BS edited and reviewed the manuscript.
The authors declare that they have no conflict of interest.
The Authors wish to thank Maciej Buczek for providing the material for the
study by permitting us to collect the data on his farm. We also thank
Piotr Błaszczyk, PhD, for his scholarly translation of the manuscript into
English.
Edited by: Manfred
Mielenz
Reviewed by: two anonymous referees
Bachtrog, D., Mank, J. E., Peichel, C. L., Kirkpatrick, M., Otto, S. P., Ashman, T.-L., Hahn, M. W., Kitano, J., Mayrose, I., Ming, R., Perrin, N., Ross, L., Valenzuela, N., and Vamosi, J. C.: The Tree of Sex Consortium, Sex determination: Why so many ways of doing it?, PLOS Biol., 12, e1001899, https://doi.org/10.1371/journal.pbio.1001899, 2014.
Bacon, S. and McClintock, M. K.: Multiple factors determine the sex ratio of postpartum-conceived Norway rat litters, Physiol. Behav., 56, 359–366, https://doi.org/10.1016/0031-9384(94)90207-0, 1994.
Bakken, M.: Sex-ratio variation and maternal investment in relation to social environment among farmed silver-fox vixens (Vulpes vulpes) of high competition capacity, J. Anim. Breed. Genet., 112, 463–468, https://doi.org/10.1111/j.1439-0388.1995.tb00584.x, 1995.
Bakken, M.: The effect of an improved man–animal relationship on sex ratio in litters and on growth and behaviour in cubs among farmed silver fox (Vulpes vulpes), Appl. Anim. Behav. Sci., 56, 309–317, https://doi.org/10.1016/S0168-1591(97)00088-9, 1998.
Barrientos, R.: Adult sex-ratio distortion in the native European polecat is related to the expansion of the invasive American mink, Biol. Conserv., 186, 28–34, https://doi.org/10.1016/j.biocon.2015.02.030, 2015.
Bonesi, L., Harrington, L. A., Maran, T., Sidorovich, V. E., and Macdonald, D. W.: Demography of three populations of American mink Mustela vison in Europe, Mammal Rev., 36, 98–106, https://doi.org/10.1111/j.1365-2907.2006.00079.x, 2016
Brzeziński, M., Jędrzejewski, W., and Jędrzejewska, B.: Winter home ranges and movements of polecats Mustela putorius in Białowieża Primeval Forest, Poland, Acta Theriol., 37, 181–191, https://doi.org/10.4098/AT.arch.92-18, 1992.
Brzeziński, M., Marzec, M., and Żmihorski, M.: Spatial distribution, activity, habitat selection of American mink (Neovison vison) and polecats (Mustela putorius) inhabiting the vicinity of eutrophic lakes in NE Poland, Folia Zool., 59, 183–191, https://doi.org/10.25225/fozo.v59.i3.a3.2010, 2010.
Cameron, E. Z.: Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: Evidence for a mechanism, P. Roy. Soc. Lond. B, 271, 1723–1728, https://doi.org/10.1098/rspb.2004.2773, 2014.
Craik, J. C. A.: Sex ratio in catches of American mink – How to catch the females, J. Nat. Conserv., 16, 56–60, https://doi.org/10.1016/j.jnc.2008.01.003, 2008.
Guillon, J.-M.: Sex ratio evolution when fitness and dispersal vary, Evol. Ecol., 30, 1097–1115, https://doi.org/10.1007/s10682-016-9869-9, 2016.
Keller, M. C., Nesse, R. M., and Hofferth, S.: The Trivers-Willard hypothesis of parental investment: No effect in the contemporary United States, Evol. Hum. Behav., 22, 343–360, https://doi.org/10.1016/S1090-5138(01)00075-7, 2001.
Krackow, S.: The developmental asynchrony hypothesis for sex ratio manipulation, J. Theor. Biol., 176, 273–280, https://doi.org/10.1006/jtbi.1995.0197, 1995a.
Krackow, S.: Potential mechanisms for sex ratio adjustment in mammals and birds, Biol. Rev. Camb. Philos., 70, 225–241, https://doi.org/10.1111/j.1469-185X.1995.tb01066.x, 1995b.
Krackow, S. and Burgoyne, P. S.: Timing of mating, developmental asynchrony and the sex ratio in mice, Physiol. Behav., 63, 81–84, https://doi.org/10.1016/S0031-9384(97)00393-4, 1998.
Meagher, R. K., Bechard, A., and Mason, G. J.: Mink with divergent activity levels have divergent reproductive strategies, Ethology, 118, 543–554, https://doi.org/10.1111/j.1439-0310.2012.02044.x, 2012.
Milki, A. A., Jun, S. H., Hinckley, M. D., Westphal, L. W., Giudice, L. C., and Behr, B.: Comparison of the sex ratio with blastocyst transfer and cleavage stage transfer, J. Assist. Reprod. Gen., 20, 323–326, https://doi.org/10.1023/A:1024861624805, 2003.
Monclús, R., Von Holst, D., Blumstein, D. T., and Rödel, H. G.: Long-term effects of liter sex ratio on female reproduction in two iteroparous mammals, Funct. Ecol., 28, 954–962, https://doi.org/10.1111/1365-2435.12231, 2014.
Orzack, S. H., Stubblefield, J. W., Akmaev, V. R., Colls, P., Munné, S., Scholl, T., Steinsaltz, D., and Zuckerman, J. E.: The human sex ratio from conception to birth, P. Natl. Acad. Sci. USA, 112, E2102–E2111, https://doi.org/10.1073/pnas.1416546112, 2015.
Price, E. O.: Animal domestication and behavior, CABI Publishing, p. 81, 2002.
Renaville, R., Haezebroeck, V., Parmentier, I., Pirard, M., Fontaine, S., and Portetelle, D.: Sex preselection in mammals, Biotechnology in Animal Husbandry, 5, 225–233, https://doi.org/10.1007/0-306-46887-5_13, 2001.
Ross, L.: Sex determination, Encyclopedia of Evolutionary Biology, 4, 81–88, https://doi.org/10.1016/B978-0-12-800049-6.00146-3, 2016.
Székely, T., Weissing, F. J., and Komdeur, J.: Adult sex ratio variation: implications for breeding system evolution, J. Evol. Biol., 27, 1500–1512, https://doi.org/10.1111/jeb.12415, 2014.
Schüttler, E., Ibarra, J. T., Gruber, B., Rozzi, R., and Jax, K.: Abundance and habitat preferences of the southernmost population of mink: implications for managing a recent island invasion, Biodivers. Conserv., 19, 725–743, https://doi.org/10.1007/s10531-009-9730-3, 2010.
Sidorovich, V. E.: Reproductive plasticity of the American mink Mustela vison in Belarus, Acta Theriol., 38, 175–183, https://doi.org/10.4098/AT.arch.93-16, 1993.
Sundell, G.: The sex ratio before uterine implantation in the golden hamster, J. Embryol. Exp. Morph., 10, 58–63, 1962.
Trut, L. N.: Sex ratio in silver foxes: effects of domestication and the star gene, Theor. Appl. Genet., 92, 109–115, https://doi.org/10.1007/BF00222959, 1996.
Vega, M. D., Peña, A. I., Gullón, J., Prieto, C., Barrio, M., Becerra, J. J., Herradón, P. G., and Quintela, L. A.: Sex ration in rabbits following modified artificial insemination, Anim. Reprod. Sci., 103, 385–391, https://doi.org/10.1016/j.anireprosci.2007.05.007, 2008.
Venge, O.: The sex ratio in farm mink, Acta Zool., 34, 293–302, https://doi.org/10.1111/j.1463-6395.1953.tb00473.x, 1953.
Zwiernik, M. J., Kay, D. P., Moore, J., Beckett, K. J., Khim, J. S., Newsted, J. L., Roark, S. A., and Giesy, J. P.: Exposure and effects assessment of resident mink (Mustela vison) exposed to polychlorinated dibenzofurans and other dioxin-like compounds in the Tittabawassee River Basin, Midland, Michigan, USA, Environ. Toxicol. Chem., 27, 2076–2087, https://doi.org/10.1897/07-489.1, 2008.